Preclinical development of a long-acting trivalent bispecific nanobody targeting IL-5 for the treatment of eosinophilic asthma
- PMID: 36403040
- PMCID: PMC9675287
- DOI: 10.1186/s12931-022-02240-1
Preclinical development of a long-acting trivalent bispecific nanobody targeting IL-5 for the treatment of eosinophilic asthma
Abstract
Background: Eosinophilic asthma is a common subtype of severe asthma with high morbidity and mortality. The cytokine IL-5 has been shown to be a key driver of the development and progression of disease. Although approved monoclonal antibodies (mAbs) targeting IL-5/IL-5R have shown good safety and efficacy, some patients have inadequate responses and frequent dosing results in medication nonadherence.
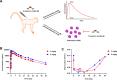
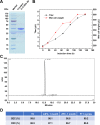
Results: We constructed a novel trivalent bispecific nanobody (Nb) consisting of 3 VHHs that bind to 2 different epitopes of IL-5 and 1 epitope of albumin derived from immunized phage display libraries. This trivalent IL-5-HSA Nb exhibited similar IL-5/IL-5R blocking activities to mepolizumab (Nucala), an approved targeting IL-5 mAb. Surprisingly, this trivalent Nb was 58 times more active than mepolizumab in inhibiting TF-1-cell proliferation. In primate studies, the trivalent IL-5-HSA Nb showed excellent pharmacokinetic properties, and peripheral blood eosinophil levels remained significantly suppressed for two months after a single dose. In addition, the trivalent IL-5-HSA Nb could be produced on a large scale in a P. pastoris X-33 yeast system with high purity and good thermal stability.
Conclusions: These findings suggest that the trivalent bispecific IL-5-HSA Nb has the potential to be a next-generation therapeutic agent targeting IL-5 for the treatment of severe eosinophilic asthma.
Keywords: Eosinophilic asthma; IL-5; Long-acting; Trivalent nanobody.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab.Drug Des Devel Ther. 2017 Oct 30;11:3137-3144. doi: 10.2147/DDDT.S150656. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29133975 Free PMC article. Review.
-
The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis.Allergol Int. 2020 Apr;69(2):178-186. doi: 10.1016/j.alit.2020.02.002. Epub 2020 Mar 2. Allergol Int. 2020. PMID: 32139163 Review.
-
Evidence for the efficacy and safety of anti-interleukin-5 treatment in the management of refractory eosinophilic asthma.Ther Adv Respir Dis. 2015 Aug;9(4):135-45. doi: 10.1177/1753465815581279. Epub 2015 Apr 21. Ther Adv Respir Dis. 2015. PMID: 25900924 Review.
-
Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys.J Allergy Clin Immunol. 2001 Aug;108(2):250-7. doi: 10.1067/mai.2001.116576. J Allergy Clin Immunol. 2001. PMID: 11496242
-
Anti-IL-5 therapies for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD013432. doi: 10.1002/14651858.CD013432.pub2. Cochrane Database Syst Rev. 2020. PMID: 33295032 Free PMC article.
Cited by
-
Identification and Characterization of a Novel Nanobody Against Human CTGF to Reveal Its Antifibrotic Effect in an in vitro Model of Liver Fibrosis.Int J Nanomedicine. 2023 Sep 21;18:5407-5422. doi: 10.2147/IJN.S428430. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37753068 Free PMC article.
-
Budesonide-incorporated inhalable lipid nanoparticles for antiTSLP nanobody mRNA delivery to treat steroid-resistant asthma.Nat Commun. 2025 Jul 1;16(1):6013. doi: 10.1038/s41467-025-61114-4. Nat Commun. 2025. PMID: 40593770 Free PMC article.
-
The potential role of nanobodies in asthma therapy.Front Pharmacol. 2025 Jan 20;15:1510806. doi: 10.3389/fphar.2024.1510806. eCollection 2024. Front Pharmacol. 2025. PMID: 39902079 Free PMC article. Review.
-
NANOBODIES®: A Review of Diagnostic and Therapeutic Applications.Int J Mol Sci. 2023 Mar 22;24(6):5994. doi: 10.3390/ijms24065994. Int J Mol Sci. 2023. PMID: 36983063 Free PMC article. Review.
-
Single-Domain Antibodies-Novel Tools to Study and Treat Allergies.Int J Mol Sci. 2024 Jul 11;25(14):7602. doi: 10.3390/ijms25147602. Int J Mol Sci. 2024. PMID: 39062843 Free PMC article. Review.
References
-
- Nelson RK, Bush A, Stokes J, Nair P, Akuthota P. Eosinophilic asthma. J Allergy Clin Immunol. 2020;8:465–473. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

