Current perspectives on diffuse midline glioma and a different role for the immune microenvironment compared to glioblastoma
- PMID: 36403059
- PMCID: PMC9675250
- DOI: 10.1186/s12974-022-02630-8
Current perspectives on diffuse midline glioma and a different role for the immune microenvironment compared to glioblastoma
Abstract
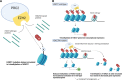
Diffuse midline glioma (DMG), formerly called diffuse intrinsic pontine glioma (DIPG), is a high-grade malignant pediatric brain tumor with a near-zero survival rate. To date, only radiation therapy provides marginal survival benefit; however, the median survival time remains less than a year. Historically, the infiltrative nature and sensitive location of the tumor rendered surgical removal and biopsies difficult and subsequently resulted in limited knowledge of the disease, as only post-mortem tissue was available. Therefore, clinical decision-making was based upon experience with the more frequent and histologically similar adult glioblastoma (GBM). Recent advances in tissue acquisition and molecular profiling revealed that DMG and GBM are distinct disease entities, with separate tissue characteristics and genetic profiles. DMG is characterized by heterogeneous tumor tissue often paired with an intact blood-brain barrier, possibly explaining its resistance to chemotherapy. Additional profiling shed a light on the origin of the disease and the influence of several mutations such as a highly recurring K27M mutation in histone H3 on its tumorigenesis. Furthermore, early evidence suggests that DMG has a unique immune microenvironment, characterized by low levels of immune cell infiltration, inflammation, and immunosuppression that may impact disease development and outcome. Within the tumor microenvironment of GBM, tumor-associated microglia/macrophages (TAMs) play a large role in tumor development. Interestingly, TAMs in DMG display distinct features and have low immune activation in comparison to other pediatric gliomas. Although TAMs have been investigated substantially in GBM over the last years, this has not been the case for DMG due to the lack of tissue for research. Bit by bit, studies are exploring the TAM-glioma crosstalk to identify what factors within the DMG microenvironment play a role in the recruitment and polarization of TAMs. Although more research into the immune microenvironment is warranted, there is evidence that targeting or stimulating TAMs and their factors provide a potential treatment option for DMG. In this review, we provide insight into the current status of DMG research, assess the knowledge of the immune microenvironment in DMG and GBM, and present recent findings and therapeutic opportunities surrounding the TAM-glioma crosstalk.
Keywords: Diffuse intrinsic pontine glioma; Diffuse midline glioma; Glioblastoma; H3K27M; Immune microenvironment; Tumor-associated microglia/macrophages.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. - PubMed
-
- Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24(8):1266–1272. - PubMed
-
- Hoffman LM, Van Zanten SEMV, Colditz N, Baugh J, Chaney B, Hoffmann M, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of Diffuse Intrinsic Pontine Glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG registries. J Clin Oncol. 2018;36(19):1963–1972. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

