Genetic deletion of Krüppel-like factor 11 aggravates traumatic brain injury
- PMID: 36403074
- PMCID: PMC9675068
- DOI: 10.1186/s12974-022-02638-0
Genetic deletion of Krüppel-like factor 11 aggravates traumatic brain injury
Abstract
Background: The long-term functional recovery of traumatic brain injury (TBI) is hampered by pathological events, such as parenchymal neuroinflammation, neuronal death, and white matter injury. Krüppel-like transcription factor 11 (KLF 11) belongs to the zinc finger family of transcription factors and actively participates in various pathophysiological processes in neurological disorders. Up to now, the role and molecular mechanisms of KLF11 in regulating the pathogenesis of brain trauma is poorly understood.
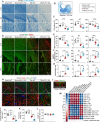
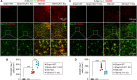
Methods: KLF11 knockout (KO) and wild-type (WT) mice were subjected to experimental TBI, and sensorimotor and cognitive functions were evaluated by rotarod, adhesive tape removal, foot fault, water maze, and passive avoidance tests. Brain tissue loss/neuronal death was examined by MAP2 and NeuN immunostaining, and Cresyl violet staining. White matter injury was assessed by Luxol fast blue staining, and also MBP/SMI32 and Caspr/Nav1.6 immunostaining. Activation of cerebral glial cells and infiltration of blood-borne immune cells were detected by GFAP, Iba-1/CD16/32, Iba-1/CD206, Ly-6B, and F4/80 immunostaining. Brian parenchymal inflammatory cytokines were measured with inflammatory array kits.
Results: Genetic deletion of KLF11 worsened brain trauma-induced sensorimotor and cognitive deficits, brain tissue loss and neuronal death, and white matter injury in mice. KLF11 genetic deficiency in mice also accelerated post-trauma astrocytic activation, promoted microglial polarization to a pro-inflammatory phenotype, and increased the infiltration of peripheral neutrophils and macrophages into the brain parenchyma. Mechanistically, loss-of-KLF11 function was found to directly increase the expression of pro-inflammatory cytokines in the brains of TBI mice.
Conclusion: KLF11 acts as a novel protective factor in TBI. KLF11 genetic deficiency in mice aggravated the neuroinflammatory responses, grey and white matter injury, and impaired long-term sensorimotor and cognitive recovery. Elucidating the functional importance of KLF11 in TBI may lead us to discover novel pharmacological targets for the development of effective therapies against brain trauma.
Keywords: Cytokines; Grey matter injury; Krüppel-like factor 11; Neurobehavioral deficits; Neuroinflammation; White matter injury.
© 2022. The Author(s).
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
-
- Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin N Am. 2020;104:213–238. - PubMed
-
- Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. - PubMed
-
- Thapa K, Khan H, Singh TG, Kaur A. Traumatic brain injury: mechanistic insight on pathophysiology and potential therapeutic targets. J Mol Neurosci. 2021;71:1725–1742. - PubMed
-
- Sato M, Chang E, Igarashi T, Noble LJ. Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain Res. 2001;917:45–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

