A novel plant-made monoclonal antibody enhances the synergetic potency of an antibody cocktail against the SARS-CoV-2 Omicron variant
- PMID: 36403203
- PMCID: PMC9946148
- DOI: 10.1111/pbi.13970
A novel plant-made monoclonal antibody enhances the synergetic potency of an antibody cocktail against the SARS-CoV-2 Omicron variant
Abstract
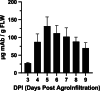
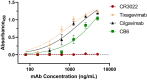
This study describes a novel, neutralizing monoclonal antibody (mAb), 11D7, discovered by mouse immunization and hybridoma generation, against the parental Wuhan-Hu-1 RBD of SARS-CoV-2. We further developed this mAb into a chimeric human IgG and recombinantly expressed it in plants to produce a mAb with human-like, highly homogenous N-linked glycans that has potential to impart greater potency and safety as a therapeutic. The epitope of 11D7 was mapped by competitive binding with well-characterized mAbs, suggesting that it is a Class 4 RBD-binding mAb that binds to the RBD outside the ACE2 binding site. Of note, 11D7 maintains recognition against the B.1.1.529 (Omicron) RBD, as well neutralizing activity. We also provide evidence that this novel mAb may be useful in providing additional synergy to established antibody cocktails, such as Evusheld™ containing the antibodies tixagevimab and cilgavimab, against the Omicron variant. Taken together, 11D7 is a unique mAb that neutralizes SARS-CoV-2 through a mechanism that is not typical among developed therapeutic mAbs and by being produced in ΔXFT Nicotiana benthamiana plants, highlights the potential of plants to be an economic and safety-friendly alternative platform for generating mAbs to address the evolving SARS-CoV-2 crisis.
Keywords: Antibody cocktail; COVID-19; Monoclonal antibody (mAb); Neutralization synergy; Plant-made antibody; Variants of Concern.
© 2022 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
M.F. is an employee of Halberd Corporation, a company that may have interest in 11D7 for possible commercial development.
Figures


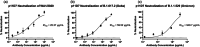
Similar articles
-
Comparative Analysis of a Human Neutralizing mAb Specific for SARS-CoV-2 Spike-RBD with Cilgavimab and Tixagevimab for the Efficacy on the Omicron Variant in Neutralizing and Detection Assays.Int J Mol Sci. 2023 Jun 13;24(12):10053. doi: 10.3390/ijms241210053. Int J Mol Sci. 2023. PMID: 37373201 Free PMC article.
-
Molecular Characterization of AZD7442 (Tixagevimab-Cilgavimab) Neutralization of SARS-CoV-2 Omicron Subvariants.Microbiol Spectr. 2023 Mar 6;11(2):e0033323. doi: 10.1128/spectrum.00333-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 36877050 Free PMC article.
-
Potential for a Plant-Made SARS-CoV-2 Neutralizing Monoclonal Antibody as a Synergetic Cocktail Component.Vaccines (Basel). 2022 May 12;10(5):772. doi: 10.3390/vaccines10050772. Vaccines (Basel). 2022. PMID: 35632528 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
Tixagevimab + cilgavimab against SARS-CoV-2: the preclinical and clinical development and real-world evidence.Expert Opin Drug Discov. 2023 Mar;18(3):231-245. doi: 10.1080/17460441.2023.2170348. Epub 2023 Jan 24. Expert Opin Drug Discov. 2023. PMID: 36649625 Review.
Cited by
-
Performance of plant-produced RBDs as SARS-CoV-2 diagnostic reagents: a tale of two plant platforms.Front Plant Sci. 2024 Jan 4;14:1325162. doi: 10.3389/fpls.2023.1325162. eCollection 2023. Front Plant Sci. 2024. PMID: 38239207 Free PMC article.
-
A genome-edited N. benthamiana line for industrial-scale production of recombinant glycoproteins with targeted N-glycosylation.Biotechnol J. 2024 Jan;19(1):e2300323. doi: 10.1002/biot.202300323. Epub 2023 Oct 15. Biotechnol J. 2024. PMID: 37804142 Free PMC article.
-
Characterization of a plant-derived monoclonal antibody targeting extracellular enveloped virions of Monkeypox virus.Front Plant Sci. 2024 Nov 1;15:1481452. doi: 10.3389/fpls.2024.1481452. eCollection 2024. Front Plant Sci. 2024. PMID: 39554528 Free PMC article.
-
Plant molecular farming: a promising frontier for orphan drug production.Biotechnol Lett. 2025 May 17;47(3):56. doi: 10.1007/s10529-025-03596-2. Biotechnol Lett. 2025. PMID: 40381123 Review.
-
A Monoclonal Antibody Produced in Glycoengineered Plants Potently Neutralizes Monkeypox Virus.Vaccines (Basel). 2023 Jun 30;11(7):1179. doi: 10.3390/vaccines11071179. Vaccines (Basel). 2023. PMID: 37514995 Free PMC article.
References
-
- Berenbaum, M.C. (1989) What is synergy? Pharmacol. Rev. 41, 93–141. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

