SARS-CoV-2 antigen tests for screening of healthcare workers; experience with over 48,000 combined antigen tests and RT-PCR tests
- PMID: 36403314
- PMCID: PMC9652101
- DOI: 10.1016/j.jcv.2022.105326
SARS-CoV-2 antigen tests for screening of healthcare workers; experience with over 48,000 combined antigen tests and RT-PCR tests
Abstract
Background: To prevent spread to patients and co-workers, health care workers (HCWs) infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) should quickly be identified. Although real time polymerase chain reaction (RT-PCR) is the gold standard, this test takes several hours, during which a HCW is unable to work. Antigen (Ag) tests may be an efficacious means of screening HCWs since they are easy to perform and provide fast results.
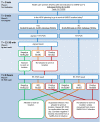
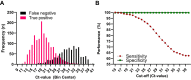
Methods: In this study, 48,010 paired results of Ag-testing and RT-PCR, performed on HCWs between January 2021 and April 2022, were evaluated to determine the diagnostic accuracy of SARS-CoV-2 Ag-tests in diagnosing potentially infectious individuals. This analysis was performed with cycling threshold values (Ct-values) ≤30 and ≤25 as cut-offs.
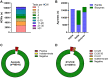
Results: Respectively 3.1% (n = 1507) and 0.3% (n = 140) of Ag-tests were positive or indeterminate, and thus indicative for SARS-CoV-2 infection. In total, 2479 (5.2%) RT-PCRs were positive, of which 1529 (61.7%) had a Ct-value ≤25 and 402 (16.2%) a Ct-value between 26 and 30. At Ct-value ≤30 as a cut-off, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of Ag-tests were 79.0%, 99.8%, 93.8% and 99.1%, respectively. At Ct-value ≤25, sensitivity further improved to 92.0%, by which the NPV increased to 99.7%.
Conclusions: To prevent transmission from HCWs to patients and co-workers, while maintaining workforce capacity, Ag-tests are a valuable addition to RT-PCR tests, as they have a quick turnaround time and excellent sensitivity for identifying individuals with high potential for transmission.
Keywords: Antigen test; Health care worker; Hospital management; Infection prevention; Real-time polymerase chain reaction; SARS-CoV-2.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interests.
Figures
Similar articles
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
Evaluation of the Abbott Panbio™ COVID-19 antigen detection rapid diagnostic test among healthcare workers in elderly care.PLoS One. 2023 Feb 24;18(2):e0276244. doi: 10.1371/journal.pone.0276244. eCollection 2023. PLoS One. 2023. PMID: 36827362 Free PMC article.
-
Rapid Antigen Test LumiraDxTM vs. Real Time Polymerase Chain Reaction for the Diagnosis of SARS-CoV-2 Infection: A Retrospective Cohort Study.Int J Environ Res Public Health. 2022 Mar 23;19(7):3826. doi: 10.3390/ijerph19073826. Int J Environ Res Public Health. 2022. PMID: 35409513 Free PMC article.
-
Usefulness of rapid antigen testing for SARS-CoV-2 screening of healthcare workers: a pilot study.Clin Exp Med. 2022 Feb;22(1):157-160. doi: 10.1007/s10238-021-00722-y. Epub 2021 May 22. Clin Exp Med. 2022. PMID: 34021827 Free PMC article.
-
COVID-19 diagnostic tests: Importance of the clinical context.Med Clin (Engl Ed). 2021 Aug 27;157(4):185-190. doi: 10.1016/j.medcle.2021.03.008. Epub 2021 Jul 31. Med Clin (Engl Ed). 2021. PMID: 34368460 Free PMC article. Review.
Cited by
-
Workplace Safety and Screening of Healthcare Workers for SARS-CoV-2 at a Tertiary Care Hospital in the Northern Emirates of United Arab Emirates.J Pharm Bioallied Sci. 2024 Jul-Sep;16(3):93-103. doi: 10.4103/jpbs.jpbs_514_24. Epub 2024 Oct 17. J Pharm Bioallied Sci. 2024. PMID: 39691541 Free PMC article.
References
-
- World Health Organisation. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int (accecced 23 August 2022).
-
- Stockholm; ECDC: 2022. European Centre for Disease Prevention and Control. Assessment of the Further Spread and Potential Impact of the SARS-CoV-2 Omicron variant of Concern in the EU/EEA, 19th Update - 27 January 2022.
-
- ECDC; Stockholm: 2022. European Centre for Disease Prevention and Control. Guidance on Ending the Isolation Period for People with COVID-19, Third update, 28 January 2022.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

