Exploring the dual effect of novel 1,4-diarylpyranopyrazoles as antiviral and anti-inflammatory for the management of SARS-CoV-2 and associated inflammatory symptoms
- PMID: 36403336
- PMCID: PMC9671780
- DOI: 10.1016/j.bioorg.2022.106255
Exploring the dual effect of novel 1,4-diarylpyranopyrazoles as antiviral and anti-inflammatory for the management of SARS-CoV-2 and associated inflammatory symptoms
Abstract
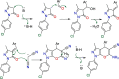
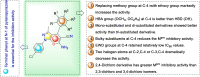
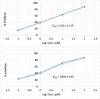
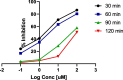
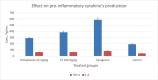
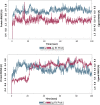
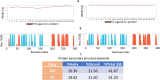
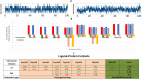
COVID-19 and associated substantial inflammations continue to threaten humankind triggering death worldwide. So, the development of new effective antiviral and anti-inflammatory medications is a major scientific goal. Pyranopyrazoles have occupied a crucial position in medicinal chemistry because of their biological importance. Here, we report the design and synthesis of a series of sixteen pyranopyrazole derivatives substituted with two aryl groups at N-1 and C-4. The designed compounds are suggested to show dual activity to combat the emerging Coronaviruses and associated substantial inflammations. All compounds were evaluated for their in vitro antiviral activity and cytotoxicity against SARS-CoV infected Vero cells. As well, the in vitro assay of all derivatives against the SARS-CoV Mpro target was performed. Results revealed the potential of three pyranopyrazoles (22, 27, and 31) to potently inhibit the viral main protease with IC50 values of 2.01, 1.83, and 4.60 μM respectively compared with 12.85 and 82.17 μM for GC-376 and lopinavir. Additionally, in vivo anti-inflammatory testing for the most active compound 27 proved its ability to reduce levels of two cytokines (TNF-α and IL-6). Molecular docking and dynamics simulation revealed consistent results with the in vitro enzymatic assay and indicated the stability of the putative complex of 27 with SARS-CoV-2 Mpro. The assessment of metabolic stability and physicochemical properties of 27 have also been conducted. This investigation identified a set of metabolically stable pyranopyrazoles as effective anti-SARS-CoV-2 Mpro and suppressors of host cell cytokine release. We believe that the new compounds deserve further chemical optimization and evaluation for COVID-19 treatment.
Keywords: Antiviral; COVID-19; Molecular docking; Molecular dynamics; Physicochemical properties; SARS-CoV-2 M(pro).
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Pimenta dioica (L.) Merr. Bioactive Constituents Exert Anti-SARS-CoV-2 and Anti-Inflammatory Activities: Molecular Docking and Dynamics, In Vitro, and In Vivo Studies.Molecules. 2021 Sep 27;26(19):5844. doi: 10.3390/molecules26195844. Molecules. 2021. PMID: 34641388 Free PMC article.
-
Synthesis of new selective agents with dual anti-inflammatory and SARS-CoV-2 Mpro inhibitory activity: Antipyrine-celecoxib hybrid analogues; COX-2, COVID-19 cytokine storm and replication inhibitory activities.Bioorg Chem. 2025 Jun 15;160:108429. doi: 10.1016/j.bioorg.2025.108429. Epub 2025 Apr 3. Bioorg Chem. 2025. PMID: 40199011
-
In vitro inhibition and molecular docking of a new ciprofloxacin-chalcone against SARS-CoV-2 main protease.Fundam Clin Pharmacol. 2022 Feb;36(1):160-170. doi: 10.1111/fcp.12708. Epub 2021 Jul 30. Fundam Clin Pharmacol. 2022. PMID: 34268806 Free PMC article.
-
Aspulvins A-H, Aspulvinone Analogues with SARS-CoV-2 Mpro Inhibitory and Anti-inflammatory Activities from an Endophytic Cladosporium sp.J Nat Prod. 2022 Apr 22;85(4):878-887. doi: 10.1021/acs.jnatprod.1c01003. Epub 2022 Mar 16. J Nat Prod. 2022. PMID: 35293744 Review.
-
Dual action anti-inflammatory/antiviral isoquinoline alkaloids as potent naturally occurring anti-SARS-CoV-2 agents: A combined pharmacological and medicinal chemistry perspective.Phytother Res. 2023 May;37(5):2168-2186. doi: 10.1002/ptr.7833. Epub 2023 Apr 11. Phytother Res. 2023. PMID: 37039761 Review.
Cited by
-
Phenylpyrazolone-1,2,3-triazole Hybrids as Potent Antiviral Agents with Promising SARS-CoV-2 Main Protease Inhibition Potential.Pharmaceuticals (Basel). 2023 Mar 20;16(3):463. doi: 10.3390/ph16030463. Pharmaceuticals (Basel). 2023. PMID: 36986562 Free PMC article.
-
Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment.Molecules. 2024 Nov 15;29(22):5403. doi: 10.3390/molecules29225403. Molecules. 2024. PMID: 39598790 Free PMC article. Review.
-
Multicomponent synthesis of pyrano-pyrazolo-pyridones by a bimetallic cobalt-cadmium magnetic catalyst.Sci Rep. 2025 Aug 12;15(1):29462. doi: 10.1038/s41598-025-15535-2. Sci Rep. 2025. PMID: 40790353 Free PMC article.
-
Recent highlights in the synthesis and biological significance of pyrazole derivatives.Heliyon. 2024 Oct 9;10(20):e38894. doi: 10.1016/j.heliyon.2024.e38894. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39492900 Free PMC article. Review.
-
Discovery of Pyrano[2,3-c]pyrazole Derivatives as Novel Potential Human Coronavirus Inhibitors: Design, Synthesis, In Silico, In Vitro, and ADME Studies.Pharmaceuticals (Basel). 2024 Feb 2;17(2):198. doi: 10.3390/ph17020198. Pharmaceuticals (Basel). 2024. PMID: 38399412 Free PMC article.
References
-
- The World Health Organization, WHO Coronavirus (COVID-19) Dashboard, (2022). https://covid19.who.int/ (accessed July 7, 2022).
-
- M.I.A. Hamed, K.M. Darwish, R. Soltane, A. Chrouda, A. Mostafa, N.M. Abo Shama, S.S. Elhady, H.S. Abulkhair, A.E. Khodir, A.A. Elmaaty, A.A. Al-karmalawy, β-Blockers bearing hydroxyethylamine and hydroxyethylene as potential SARS-CoV-2 Mpro inhibitors: rational based design, in silico , in vitro , and SAR studies for lead optimization, RSC Adv. 11 (2021) 35536–35558. 10.1039/D1RA04820A. - PMC - PubMed
-
- Fu L., Ye F., Feng Y., Yu F., Wang Q., Wu Y., Zhao C., Sun H., Huang B., Niu P., Song H., Shi Y., Li X., Tan W., Qi J., Gao G.F. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Commun. 2020;11:4417. doi: 10.1038/s41467-020-18233-x. - DOI - PMC - PubMed
-
- Owen D.R., Allerton C.M.N., Anderson A.S., Aschenbrenner L., Avery M., Berritt S., Boras B., Cardin R.D., Carlo A., Coffman K.J., Dantonio A., Di L., Eng H., Ferre R., Gajiwala K.S., Gibson S.A., Greasley S.E., Hurst B.L., Kadar E.P., Kalgutkar A.S., Lee J.C., Lee J., Liu W., Mason S.W., Noell S., Novak J.J., Obach R.S., Ogilvie K., Patel N.C., Pettersson M., Rai D.K., Reese M.R., Sammons M.F., Sathish J.G., Singh R.S.P., Steppan C.M., Stewart A.E., Tuttle J.B., Updyke L., Verhoest P.R., Wei L., Yang Q., Zhu Y. An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19. Science (80-.) 2021;374:1586–1593. doi: 10.1126/science.abl4784. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

