Nucleoside analogs for management of respiratory virus infections: mechanism of action and clinical efficacy
- PMID: 36403338
- PMCID: PMC9671222
- DOI: 10.1016/j.coviro.2022.101279
Nucleoside analogs for management of respiratory virus infections: mechanism of action and clinical efficacy
Abstract
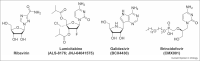
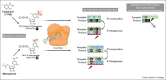
The COVID-19 pandemic has accelerated the development of nucleoside analogs to treat respiratory virus infections, with remdesivir being the first compound to receive worldwide authorization and three other nucleoside analogs (i.e. favipiravir, molnupiravir, and bemnifosbuvir) in the pipeline. Here, we summarize the current knowledge concerning their clinical efficacy in suppressing the virus and reducing the need for hospitalization or respiratory support. We also mention trials of favipiravir and lumicitabine, for influenza and respiratory syncytial virus, respectively. Besides, we outline how nucleoside analogs interact with the polymerases of respiratory viruses, to cause lethal virus mutagenesis or disturbance of viral RNA synthesis. In this way, we aim to convey the key findings on this rapidly evolving class of respiratory virus medication.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures

References
-
- Mazur N.I., Martinón-Torres F., Baraldi E., Fauroux B., Greenough A., Heikkinen T., Manzoni P., Mejias A., Nair H., Papadopoulos N.G., et al. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015;3:888–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

