Quality-of-life outcomes in older patients with early-stage rectal cancer receiving organ-preserving treatment with hypofractionated short-course radiotherapy followed by transanal endoscopic microsurgery (TREC): non-randomised registry of patients unsuitable for total mesorectal excision
- PMID: 36403589
- PMCID: PMC9722406
- DOI: 10.1016/S2666-7568(22)00239-2
Quality-of-life outcomes in older patients with early-stage rectal cancer receiving organ-preserving treatment with hypofractionated short-course radiotherapy followed by transanal endoscopic microsurgery (TREC): non-randomised registry of patients unsuitable for total mesorectal excision
Erratum in
-
Correction to Lancet Healthy Longev 2022; 3: e825-38.Lancet Healthy Longev. 2022 Dec;3(12):e816. doi: 10.1016/S2666-7568(22)00275-6. Epub 2022 Nov 23. Lancet Healthy Longev. 2022. PMID: 36435195 Free PMC article. No abstract available.
Abstract
Background: Older patients with early-stage rectal cancer are under-represented in clinical trials and, therefore, little high-quality data are available to guide treatment in this patient population. The TREC trial was a randomised, open-label feasibility study conducted at 21 centres across the UK that compared organ preservation through short-course radiotherapy (SCRT; 25 Gy in five fractions) plus transanal endoscopic microsurgery (TEM) with standard total mesorectal excision in adults with stage T1-2 rectal adenocarcinoma (maximum diameter ≤30 mm) and no lymph node involvement or metastasis. TREC incorporated a non-randomised registry offering organ preservation to patients who were considered unsuitable for total mesorectal excision by the local colorectal cancer multidisciplinary team. Organ preservation was achieved in 56 (92%) of 61 non-randomised registry patients with local recurrence-free survival of 91% (95% CI 84-99) at 3 years. Here, we report acute and long-term patient-reported outcomes from this non-randomised registry group.
Methods: Patients considered by the local colorectal cancer multidisciplinary team to be at high risk of complications from total mesorectal excision on the basis of frailty, comorbidities, and older age were included in a non-randomised registry to receive organ-preserving treatment. These patients were invited to complete questionnaires on patient-reported outcomes (the European Organisation for Research and Treatment of Cancer Quality of Life [EORTC-QLQ] questionnaire core module [QLQ-C30] and colorectal cancer module [QLQ-CR29], the Colorectal Functional Outcome [COREFO] questionnaire, and EuroQol-5 Dimensions-3 Level [EQ-5D-3L]) at baseline and at months 3, 6, 12, 24, and 36 postoperatively. To aid interpretation, data from patients in the non-randomised registry were compared with data from those patients in the TREC trial who had been randomly assigned to organ-preserving therapy, and an additional reference cohort of aged-matched controls from the UK general population. This study is registered with the ISRCTN registry, ISRCTN14422743, and is closed.
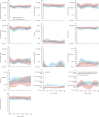
Findings: Between July 21, 2011, and July 15, 2015, 88 patients were enrolled onto the TREC study to undergo organ preservation, of whom 27 (31%) were randomly allocated to organ-preserving therapy and 61 (69%) were added to the non-randomised registry for organ-preserving therapy. Non-randomised patients were older than randomised patients (median age 74 years [IQR 67-80] vs 65 years [61-71]). Organ-preserving treatment was well tolerated among patients in the non-randomised registry, with mild worsening of fatigue; quality of life; physical, social, and role functioning; and bowel function 3 months postoperatively compared with baseline values. By 6-12 months, most scores had returned to baseline values, and were indistinguishable from data from the reference cohort. Only mild symptoms of faecal incontinence and urgency, equivalent to less than one episode per week, persisted at 36 months among patients in both groups.
Interpretation: The SCRT and TEM organ-preservation approach was well tolerated in older and frailer patients, showed good rates of organ preservation, and was associated with low rates of acute and long-term toxicity, with minimal effects on quality of life and functional status. Our findings support the adoption of this approach for patients considered to be at high risk from radical surgery.
Funding: Cancer Research UK.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AG is funded by a Cancer Research UK clinical trial fellowship (CRUK/28301). PQ and NPW were funded by a programme grant from Yorkshire Cancer Research. PQ is a National Institute for Health and Care Research senior investigator. NPW reports personal fees from Eisai. KB reports stock ownership in GlaxoSmithKline and UCB. All other authors declare no competing interests.
Figures

Comment in
-
A watch-and-wait strategy or local excision in complete clinical responders after radiation for early-stage rectal cancer.Lancet Healthy Longev. 2022 Dec;3(12):e807-e808. doi: 10.1016/S2666-7568(22)00240-9. Epub 2022 Nov 17. Lancet Healthy Longev. 2022. PMID: 36403588 No abstract available.
References
-
- Gollins S, Moran B, Adams R, et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): guidelines for the management of cancer of the colon, rectum and anus (2017) - multidisciplinary management. Colorectal Dis. 2017;19(suppl 1):37–66. - PubMed
-
- Rutten HJT, den Dulk M, Lemmens VEPP, van de Velde CJH, Marijnen CAM. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol. 2008;9:494–501. - PubMed
-
- de Neree Tot Babberich MPM, Detering R, Dekker JWT, et al. Achievements in colorectal cancer care during 8 years of auditing in the Netherlands. Eur J Surg Oncol. 2018;44:1361–1370. - PubMed

