Dihydromyricetin supplementation during in vitro culture improves porcine oocyte developmental competence by regulating oxidative stress
- PMID: 36403957
- PMCID: PMC9939282
- DOI: 10.1262/jrd.2022-031
Dihydromyricetin supplementation during in vitro culture improves porcine oocyte developmental competence by regulating oxidative stress
Abstract
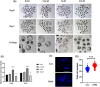
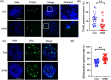
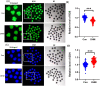
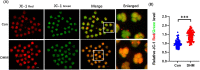
Dihydromyricetin (DHM), a dihydroflavonoid compound, exhibits a variety of biological activities, including antitumor activity. However, the effects of DHM on mammalian reproductive processes, especially during early embryonic development, remain unclear. In this study, we added DHM to porcine zygotic medium to explore the influence and underlying mechanisms of DHM on the developmental competence of parthenogenetically activated porcine embryos. Supplementation with 5 μM DHM during in vitro culture (IVC) significantly improved blastocyst formation rate and increased the total number of cells in porcine embryos. Further, DHM supplementation also improved glutathione levels and mitochondrial membrane potential; reduced natural reactive oxygen species levels in blastomeres and apoptosis rate; upregulated Nanog, Oct4, SOD1, SOD2, Sirt1, and Bcl2 expression; and downregulated Beclin1, ATG12, and Bax expression. Collectively, DHM supplementation regulated oxidative stress during IVC and could act as a potential antioxidant during in vitro porcine oocytes maturation.
Keywords: Dihydromyricetin; In vitro culture; Oxidative stress; Porcine oocyte.
Conflict of interest statement
The authors have no conflicts to declare.
Figures
Similar articles
-
α-Ketoglutarate Improves Meiotic Maturation of Porcine Oocytes and Promotes the Development of PA Embryos, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway.Oxid Med Cell Longev. 2022 Feb 21;2022:7113793. doi: 10.1155/2022/7113793. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35237383 Free PMC article.
-
Supplementation with asiatic acid during in vitro maturation improves porcine oocyte developmental competence by regulating oxidative stress.Theriogenology. 2021 Sep 15;172:169-177. doi: 10.1016/j.theriogenology.2021.06.013. Epub 2021 Jun 17. Theriogenology. 2021. PMID: 34174755
-
Carnosic acid improves porcine early embryonic development by inhibiting the accumulation of reactive oxygen species.J Reprod Dev. 2020 Dec 22;66(6):555-562. doi: 10.1262/jrd.2020-086. Epub 2020 Oct 14. J Reprod Dev. 2020. PMID: 33055461 Free PMC article.
-
Multiple molecular and cellular mechanisms of the antitumour effect of dihydromyricetin (Review).Biomed Rep. 2024 Mar 26;20(5):82. doi: 10.3892/br.2024.1769. eCollection 2024 May. Biomed Rep. 2024. PMID: 38628627 Free PMC article. Review.
-
Dihydromyricetin: an emerging compound with comprehensive effects on multiple systems.Front Pharmacol. 2025 Jan 3;15:1488003. doi: 10.3389/fphar.2024.1488003. eCollection 2024. Front Pharmacol. 2025. PMID: 39830336 Free PMC article. Review.
Cited by
-
METTL7A improves bovine IVF embryo competence by attenuating oxidative stress.bioRxiv [Preprint]. 2024 Dec 17:2024.12.17.628915. doi: 10.1101/2024.12.17.628915. bioRxiv. 2024. Update in: Biol Reprod. 2025 Apr 13;112(4):628-639. doi: 10.1093/biolre/ioaf018. PMID: 39763908 Free PMC article. Updated. Preprint.
-
METTL7A improves bovine IVF embryo competence by attenuating oxidative stress†.Biol Reprod. 2025 Apr 13;112(4):628-639. doi: 10.1093/biolre/ioaf018. Biol Reprod. 2025. PMID: 39883095
References
-
- Fowler KE, Mandawala AA, Griffin DK, Walling GA, Harvey SC. The production of pig preimplantation embryos in vitro: Current progress and future prospects. Reprod Biol 2018; 18: 203–211. - PubMed
-
- Ouyang H, Han J, Huang Y. Pig cloning using somatic cell nuclear transfer. Methods Mol Biol 2021; 2239: 1–18. - PubMed
-
- Soto-Heras S, Paramio MT. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res Vet Sci 2020; 132: 342–350. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

