An effective and chemotherapy-free strategy of all-trans retinoic acid and arsenic trioxide for acute promyelocytic leukemia in all risk groups (APL15 trial)
- PMID: 36404343
- PMCID: PMC9676182
- DOI: 10.1038/s41408-022-00753-y
An effective and chemotherapy-free strategy of all-trans retinoic acid and arsenic trioxide for acute promyelocytic leukemia in all risk groups (APL15 trial)
Abstract
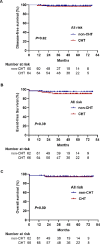
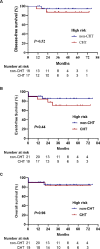
The combination of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) has been demonstrated to have comparable effectiveness or better to ATRA and chemotherapy (CHT) in non-high-risk acute promyelocytic leukemia (APL). However, the efficacy of ATRA-ATO compared to ATRA-ATO plus CHT in high-risk APL remains unknown. Here we performed a randomized multi-center non-inferiority phase III study to compare the efficacy of ATRA-ATO and ATRA-ATO plus CHT in newly diagnosed all-risk APL to address this question. Patients were assigned to receive ATRA-ATO for induction, consolidation, and maintenance or ATRA-ATO plus CHT for induction followed by three cycles of consolidation therapy, and maintenance therapy with ATRA-ATO. In the non-CHT group, hydroxyurea was used to control leukocytosis. A total of 128 patients were treated. The complete remission rate was 97% in both groups. The 2-year disease-free, event-free survival rates in the non-CHT group and CHT group in all-risk patients were 98% vs 97%, and 95% vs 92%, respectively (P = 0.62 and P = 0.39, respectively). And they were 94% vs 87%, and 85% vs 78% in the high-risk patients (P = 0.52 and P = 0.44, respectively). This study demonstrated that ATRA-ATO had the same efficacy as the ATRA-ATO plus CHT in the treatment of patients with all-risk APL.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Improved Outcomes With Retinoic Acid and Arsenic Trioxide Compared With Retinoic Acid and Chemotherapy in Non-High-Risk Acute Promyelocytic Leukemia: Final Results of the Randomized Italian-German APL0406 Trial.J Clin Oncol. 2017 Feb 20;35(6):605-612. doi: 10.1200/JCO.2016.67.1982. Epub 2016 Oct 31. J Clin Oncol. 2017. PMID: 27400939 Clinical Trial.
-
Arsenic trioxide and all-trans retinoic acid (ATRA) treatment for acute promyelocytic leukemia in all risk groups: study protocol for a randomized controlled trial.Trials. 2018 Sep 5;19(1):476. doi: 10.1186/s13063-018-2812-3. Trials. 2018. PMID: 30185214 Free PMC article.
-
PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy.Leukemia. 2016 Oct;30(10):1987-1992. doi: 10.1038/leu.2016.122. Epub 2016 May 2. Leukemia. 2016. PMID: 27133819 Clinical Trial.
-
Chemotherapy-free approaches to newly-diagnosed acute promyelocytic leukaemia: is oral-arsenic trioxide/all-trans retinoic acid/ascorbic acid the answer?Expert Rev Hematol. 2024 Oct;17(10):661-667. doi: 10.1080/17474086.2024.2391098. Epub 2024 Aug 12. Expert Rev Hematol. 2024. PMID: 39120131 Review.
-
Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy.Philos Trans R Soc Lond B Biol Sci. 2007 Jun 29;362(1482):959-71. doi: 10.1098/rstb.2007.2026. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17317642 Free PMC article. Review.
Cited by
-
Hepatotoxicity induced by arsenic trioxide: clinical features, mechanisms, preventive and potential therapeutic strategies.Front Pharmacol. 2025 Feb 20;16:1536388. doi: 10.3389/fphar.2025.1536388. eCollection 2025. Front Pharmacol. 2025. PMID: 40051569 Free PMC article. Review.
-
The genomic landscape of sensitivity to arsenic trioxide uncovered by genome-wide CRISPR-Cas9 screening.Front Oncol. 2023 May 12;13:1178686. doi: 10.3389/fonc.2023.1178686. eCollection 2023. Front Oncol. 2023. PMID: 37251921 Free PMC article.
-
Acute Promyelocytic Leukemia: Review of Complications Related to All-Trans Retinoic Acid and Arsenic Trioxide Therapy.Cancers (Basel). 2024 Mar 15;16(6):1160. doi: 10.3390/cancers16061160. Cancers (Basel). 2024. PMID: 38539495 Free PMC article. Review.
-
Acute leukemias and complicated lymphomas: pearls to optimize management when patients stay local.Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):311-317. doi: 10.1182/hematology.2023000430. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066909 Free PMC article.
-
Patients with high-risk acute promyelocytic leukemia need maintenance therapy for 1 year - the PROS.Haematologica. 2025 Jul 1;110(7):1454-1458. doi: 10.3324/haematol.2025.287417. Epub 2025 Apr 3. Haematologica. 2025. PMID: 40176767 Free PMC article. No abstract available.
References
-
- Avvisati G, Lo Coco F, Diverio D, Falda M, Ferrara F, Lazzarino M, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) pilot study. Blood. 1996;88:1390–8. doi: 10.1182/blood.V88.4.1390.bloodjournal8841390. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

