Lowering fasting blood glucose with non-dialyzable material of cranberry extract is dependent on host genetic background, sex and diet
- PMID: 36404387
- PMCID: PMC10272894
- DOI: 10.1002/ame2.12291
Lowering fasting blood glucose with non-dialyzable material of cranberry extract is dependent on host genetic background, sex and diet
Abstract
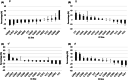
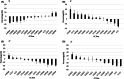
Background: Type 2 diabetes (T2D) is a polygenic metabolic disease, characterized by high fasting blood glucose (FBG). The ability of cranberry (CRN) fruit to regulate glycemia in T2D patients is well known. Here, a cohort of 13 lines of the genetically diverse Collaborative Cross (CC) mouse model was assessed for the effect of non-dialyzable material (NDM) of cranberry extract in lowering fasting blood glucose.
Methods: Eight-week-old mice were maintained on either a standard chow diet (control group) or a high-fat diet (HFD) for 12 weeks, followed by injections of intraperitoneal (IP) NDM (50 mg/kg) per mouse, three times a week for the next 6 weeks. Absolute FBG (mg/dl) was measured bi-weekly and percentage changes in FBG (%FBG) between weeks 0 and 12 were calculated.
Results: Statistical analysis showed a significant decrease in FBG between weeks 0 and 12 in male and female mice maintained on CHD. However, a non-significant increase in FBG values was observed in male and female mice maintained on HFD during the same period. Following administration of NDM during the following 6 weeks, the results show a variation in significant levels of FBG lowering between lines, male and female mice and under the different diets.
Conclusion: The results suggest that the efficacy of NDM treatment in lowering FGB depends on host genetic background (pharmacogenetics), sex of the mouse (pharmacosex), and diet (pharmacodiet). All these results support the need for follow-up research to better understand and implement a personalized medicine approach/utilization of NDM for reducing FBG.
Keywords: chow diet (CHD); collaborative cross (CC) mouse model; fasting blood glucose (FBG); high-fat diet (HFD); non-dialyzable material (NDM) of cranberry extract; type 2 diabetes (T2D).
© 2022 The Authors. Animal Models and Experimental Medicine published by John Wiley & Sons Australia, Ltd on behalf of The Chinese Association for Laboratory Animal Sciences.
Conflict of interest statement
None.
Figures

Similar articles
-
Studying the pharmacogenomic effect of cranberry extract on reducing body weight using collaborative cross mice.Food Funct. 2021 Jun 7;12(11):4972-4982. doi: 10.1039/d0fo02865g. Epub 2021 Apr 15. Food Funct. 2021. PMID: 34100468
-
High-fat-diet induced development of increased fasting glucose levels and impaired response to intraperitoneal glucose challenge in the collaborative cross mouse genetic reference population.BMC Genet. 2016 Jan 5;17:10. doi: 10.1186/s12863-015-0321-x. BMC Genet. 2016. PMID: 26728312 Free PMC article.
-
Unraveling the Host Genetic Background Effect on Internal Organ Weight Influenced by Obesity and Diabetes Using Collaborative Cross Mice.Int J Mol Sci. 2023 May 3;24(9):8201. doi: 10.3390/ijms24098201. Int J Mol Sci. 2023. PMID: 37175908 Free PMC article.
-
Intestinal cancer development in response to oral infection with high-fat diet-induced Type 2 diabetes (T2D) in collaborative cross mice under different host genetic background effects.Mamm Genome. 2023 Mar;34(1):56-75. doi: 10.1007/s00335-023-09979-y. Epub 2023 Feb 9. Mamm Genome. 2023. PMID: 36757430
-
Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: what is the best choice?Nutr Diabetes. 2020 Jul 2;10(1):24. doi: 10.1038/s41387-020-0127-4. Nutr Diabetes. 2020. PMID: 32616730 Free PMC article. Review.
Cited by
-
Screening for urinary markers predicting hematopoietic stem cell injury induced by busulfan using genetically diverse mice.Animal Model Exp Med. 2023 Apr;6(2):146-154. doi: 10.1002/ame2.12320. Epub 2023 Apr 16. Animal Model Exp Med. 2023. PMID: 37062934 Free PMC article.
-
Towards system genetics analysis of head and neck squamous cell carcinoma using the mouse model, cellular platform, and clinical human data.Animal Model Exp Med. 2023 Dec;6(6):537-558. doi: 10.1002/ame2.12367. Epub 2023 Dec 21. Animal Model Exp Med. 2023. PMID: 38129938 Free PMC article. Review.
References
-
- Scheen AJ. Pharmacodynamics, efficacy and safety of sodium–glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33‐59. - PubMed
-
- Chong WH, Yanoff LB, Andraca‐Carrera E, Hai MT. Assessing the safety of glucose‐lowering drugs—a new focus for the FDA. NEJM. 2020;383:1199‐1202. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

