Nestin+ Peyer's patch resident MSCs enhance healing of inflammatory bowel disease through IL-22-mediated intestinal epithelial repair
- PMID: 36404603
- PMCID: PMC9890526
- DOI: 10.1111/cpr.13363
Nestin+ Peyer's patch resident MSCs enhance healing of inflammatory bowel disease through IL-22-mediated intestinal epithelial repair
Abstract
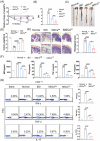
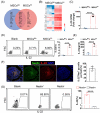
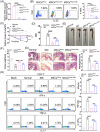
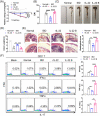
Inflammatory bowel disease (IBD) is a chronic condition characterized by gastrointestinal tract inflammation and still lacks satisfactory treatments. Mesenchymal stromal cells (MSCs) show promising potential for treating IBD, but their therapeutic efficacy varies depending on the tissue of origin. We aim to investigate whether intestine Peyer's patch (PP)-derived MSCs have superior immunomodulatory effects on T cells and better therapeutic effects on IBD compared with bone marrow-derived MSCs. We isolated PPs-derived Nestin+ MSCs (MSCsPP ) and bone marrow-derived Nestin+ MSCs (MSCsBM ) from Nestin-GFP transgenic mice to explore their curative effects on murine IBD model. Moreover, we tested the effects of IL-22 knockdown and IL-22 overexpression on the therapeutic efficacy of MSCsPP and MSCsBM in murine IBD, respectively. We demonstrated that Nestin+ cells derived from murine PPs exhibit MSC-like biological characteristics. Compared with MSCsBM , MSCsPP possess enhanced immunoregulatory ability to suppress T cell proliferation and inflammatory cytokine production. Moreover, we observed that MSCsPP exhibited greater therapeutic efficacy than MSCsBM in murine IBD models. Interestingly, IL-22, which was highly expressed in MSCsPP , could alleviate the severity of the intestinal inflammation, while knockdown IL-22 of MSCsPP remarkably weakened the therapeutic effects. More importantly, IL-22 overexpressing MSCsBM could significantly improve the symptoms of murine IBD models. This study systemically demonstrated that murine MSCsPP have a prominent advantage in murine IBD treatment, partly through IL-22.
© 2022 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy.Eur J Med Res. 2023 Jan 27;28(1):47. doi: 10.1186/s40001-023-01008-7. Eur J Med Res. 2023. PMID: 36707899 Free PMC article. Review.
-
Lack of IFN-γ response of human uterine myometrium-derived MSCs significantly improve multiple IBD parameters compared to bone marrow MSCs: Implications for anti-TNFα-refractory patients.Pharmacol Res. 2025 May;215:107716. doi: 10.1016/j.phrs.2025.107716. Epub 2025 Mar 26. Pharmacol Res. 2025. PMID: 40154933
-
Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses.World J Gastroenterol. 2013 Aug 7;19(29):4702-17. doi: 10.3748/wjg.v19.i29.4702. World J Gastroenterol. 2013. PMID: 23922467 Free PMC article.
-
Nestin+ Mesenchymal Precursors Generate Distinct Spleen Stromal Cell Subsets and Have Immunomodulatory Function.Int J Mol Sci. 2022 Oct 5;23(19):11819. doi: 10.3390/ijms231911819. Int J Mol Sci. 2022. PMID: 36233119 Free PMC article.
-
"Remodeling the intestinal immune microenvironment": immune regulation and tissue regeneration by mesenchymal stem/stromal cells in the repair microenvironment of inflammatory bowel disease.Front Immunol. 2025 May 13;16:1543702. doi: 10.3389/fimmu.2025.1543702. eCollection 2025. Front Immunol. 2025. PMID: 40433382 Free PMC article. Review.
Cited by
-
The NLRP3 molecule influences the therapeutic effects of mesenchymal stem cells through Glut1-mediated energy metabolic reprogramming.J Adv Res. 2024 Nov;65:125-136. doi: 10.1016/j.jare.2023.12.006. Epub 2023 Dec 7. J Adv Res. 2024. PMID: 38070595 Free PMC article.
-
Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells.Stem Cell Rev Rep. 2023 Jul;19(5):1214-1231. doi: 10.1007/s12015-023-10539-9. Epub 2023 Apr 14. Stem Cell Rev Rep. 2023. PMID: 37058201 Free PMC article. Review.
-
Key Interleukins in Inflammatory Bowel Disease-A Review of Recent Studies.Int J Mol Sci. 2024 Dec 26;26(1):121. doi: 10.3390/ijms26010121. Int J Mol Sci. 2024. PMID: 39795980 Free PMC article. Review.
-
Challenges and opportunities in inflammatory bowel disease: from current therapeutic strategies to organoid-based models.Inflamm Res. 2024 Apr;73(4):541-562. doi: 10.1007/s00011-024-01854-z. Epub 2024 Feb 12. Inflamm Res. 2024. PMID: 38345635 Review.
-
Distribution, contribution and regulation of nestin+ cells.J Adv Res. 2024 Jul;61:47-63. doi: 10.1016/j.jare.2023.08.013. Epub 2023 Aug 28. J Adv Res. 2024. PMID: 37648021 Free PMC article. Review.
References
-
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205‐217. - PubMed
-
- Ng SC, Kaplan GG, Tang W, et al. Population density and risk of inflammatory bowel disease: a prospective population‐based study in 13 countries or regions in Asia‐Pacific. Am J Gastroenterol. 2019;114(1):107‐115. - PubMed
-
- Dignass A, Eliakim R, Magro F, et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6(10):965‐990. - PubMed
MeSH terms
Substances
Grants and funding
- 2019B020234001/Key Research and Development Program of Guangdong Province
- 2019B020236002/Key Research and Development Program of Guangdong Province
- 2019B020235002/Key Research and Development Program of Guangdong Province
- 201803040011/Key Scientific and Technological Program of Guangzhou City
- 2018YFA0107203/National Key Research and Development Program of China, Stem Cell and Translational Research
- 2019YFA0110300/National Key Research and Development Program of China, Stem Cell and Translational Research
- 32130046/National Natural Science Foundation of China
- 81730005/National Natural Science Foundation of China
- 31771616/National Natural Science Foundation of China
- 81900075/National Natural Science Foundation of China
- 82170540/National Natural Science Foundation of China
- 2018A0303130305/Natural Science Foundation of Guangdong Province
- 2021A1515011759/Natural Science Foundation of Guangdong Province
- 201906010095/Pearl River S&T Nova Program of Guangzhou
- 2017-L163/Pioneering talents project of Guangzhou Development Zone
LinkOut - more resources
Full Text Sources
Miscellaneous

