Neutrophils are gatekeepers of mucosal immunity
- PMID: 36404627
- PMCID: PMC10496120
- DOI: 10.1111/imr.13171
Neutrophils are gatekeepers of mucosal immunity
Abstract
Mucosal tissues are constantly exposed to the outside environment. They receive signals from the commensal microbiome and tissue-specific triggers including alimentary and airborne elements and are tasked to maintain balance in the absence of inflammation and infection. Here, we present neutrophils as sentinel cells in mucosal immunity. We discuss the roles of neutrophils in mucosal homeostasis and overview clinical susceptibilities in patients with neutrophil defects. Finally, we present concepts related to specification of neutrophil responses within specific mucosal tissue microenvironments.
Keywords: gastrointestinal; lung; mucosal immunity; neutropenia; neutrophils; oral.
© 2022 John Wiley & Sons Ltd. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

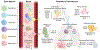
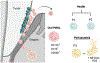

Similar articles
-
Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases.Immunol Lett. 2004 May 15;93(2-3):97-108. doi: 10.1016/j.imlet.2004.02.005. Immunol Lett. 2004. PMID: 15158604 Review.
-
The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms.Mucosal Immunol. 2014 May;7(3):455-66. doi: 10.1038/mi.2014.11. Epub 2014 Feb 26. Mucosal Immunol. 2014. PMID: 24569801 Review.
-
Ocular mucosal homeostasis of teleost fish provides insight into the coevolution between microbiome and mucosal immunity.Microbiome. 2024 Jan 13;12(1):10. doi: 10.1186/s40168-023-01716-6. Microbiome. 2024. PMID: 38218870 Free PMC article.
-
Tissue-Specific Immunity at the Oral Mucosal Barrier.Trends Immunol. 2018 Apr;39(4):276-287. doi: 10.1016/j.it.2017.08.005. Epub 2017 Sep 8. Trends Immunol. 2018. PMID: 28923364 Free PMC article. Review.
-
The Neutrophil: Constant Defender and First Responder.Front Immunol. 2020 Sep 24;11:571085. doi: 10.3389/fimmu.2020.571085. eCollection 2020. Front Immunol. 2020. PMID: 33072112 Free PMC article. Review.
Cited by
-
The emerging role of neutrophil extracellular traps in autoimmune and autoinflammatory diseases.MedComm (2020). 2025 Mar 6;6(3):e70101. doi: 10.1002/mco2.70101. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40060194 Free PMC article. Review.
-
Neutrophils and neutrophil extracellular traps in oral health and disease.Exp Mol Med. 2024 May;56(5):1055-1065. doi: 10.1038/s12276-024-01219-w. Epub 2024 May 1. Exp Mol Med. 2024. PMID: 38689085 Free PMC article. Review.
-
Hyperglycemia-Enhanced Neutrophil Extracellular Traps Drive Mucosal Immunopathology at the Oral Barrier.Adv Sci (Weinh). 2024 Dec;11(47):e2407346. doi: 10.1002/advs.202407346. Epub 2024 Nov 5. Adv Sci (Weinh). 2024. PMID: 39499780 Free PMC article.
-
Prevalence of neutropenia in the U.S. among reproductive-aged women: a population-based analysis of NHANES 2013-2020.BMC Public Health. 2025 Jan 16;25(1):181. doi: 10.1186/s12889-025-21330-5. BMC Public Health. 2025. PMID: 39819597 Free PMC article.
-
The innate immune brakes of the lung.Front Immunol. 2023 Jan 27;14:1111298. doi: 10.3389/fimmu.2023.1111298. eCollection 2023. Front Immunol. 2023. PMID: 36776895 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

