Nanoencapsulation and bioaccessibility of polyphenols of aqueous extracts from Bauhinia forficata link
- PMID: 36404894
- PMCID: PMC9672949
- DOI: 10.1016/j.fochms.2022.100144
Nanoencapsulation and bioaccessibility of polyphenols of aqueous extracts from Bauhinia forficata link
Abstract
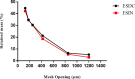
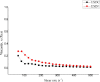
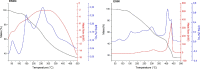
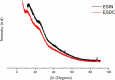
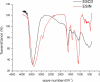
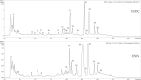
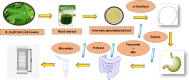
Bauhinia forficata Link is a plant rich in polyphenols that has been used mainly for its hypoglycemic activity, which is related to its antioxidant and anti-inflammatory potential. However, the beneficial effect of these bioactive compounds is directly dependent on their bioaccessibility and bioavailability, requiring processing techniques that can improve and preserve their biological activities. This work aimed to obtain nanocapsulated extracts from the infusion (ESIN) and decoction (ESDC) of B. forficata Link leaves, by spray drying. The encapsulating agents used were maltodextrin and colloidal silicon dioxide. The nanocapsules were characterized by HPLC-PDA-ESI-IT-MS n , evaluated the bioaccessibility of polyphenols after simulated digestion and their antioxidant activity. Additionally, an extensive physicochemical characterization of the nanocapsulated extracts was carried out and their stability and technological parameters were evaluated. The ESIN and ESDC extracts had yields of 57.3 % and 62.7 %, with average nanocapsules sizes of 0.202 μm and 0.179 μm, low humidity and water activity (<0.5), powder density and proper flow properties (Hausner ratio ≤ 1.25; Carr index 18-19 %). Scanning electron microscopy showed a spherical and amorphous morphology and low viscosity, which may have favored the solubility profile. The phenolic compounds of the nanocapsules degraded after 400 °C, showing high thermal stability. The infrared spectra identified the presence of maltodextrin and phenolic compounds and that there were no reactions between them. Chromatography confirmed the presence of phenolic compounds, mainly flavonols and their O-glycosylated derivatives, as well as carbohydrates, probably maltodextrin. Simulated in vitro digestion showed that polyphenols and flavonoids from ESIN and ESDC nanocapsules were bioaccessible after the gastric phase (49.38 % and 64.17 % of polyphenols and 64.08 % and 36.61 % of flavonoids) and duodenal (52.68 % and 79.06 % of polyphenols and 13.24 % and 139.03 % of flavoids), with a variation from 52.27 % to 70.55 % of the antioxidant activity maintained, by the ORAC method, after gastric digestion and still 25 %, after duodenal. Therefore, the nanoencapsulation of extracts of B. forficata is a viable option for the preservation of their bioactive compounds, making them bioaccessible and with antioxidant activity, which make them suitable for incorporation into various nutraceutical formulations, such as capsules, tablets and sachets.
Keywords: Bioactive compounds; ORAC; Phenolic compounds; Simulated digestion; Spray drying.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Spray drying encapsulation of stevia extract with maltodextrin and evaluation of the physicochemical and functional properties of produced powders.J Food Sci. 2020 Oct;85(10):3590-3600. doi: 10.1111/1750-3841.15437. Epub 2020 Sep 5. J Food Sci. 2020. PMID: 32888354
-
Hypoglycemic activity of dried extracts of Bauhinia forficata Link.Phytomedicine. 2010 Jan;17(1):37-41. doi: 10.1016/j.phymed.2009.06.007. Epub 2009 Jul 3. Phytomedicine. 2010. PMID: 19577450
-
Bauhinia forficata Link authenticity using flavonoids profile: relation with their biological properties.Food Chem. 2012 Sep 15;134(2):894-904. doi: 10.1016/j.foodchem.2012.02.201. Epub 2012 Mar 10. Food Chem. 2012. PMID: 23107705
-
Antioxidant Activity of Encapsulated Extracts and Bioactives from Natural Sources.Curr Pharm Des. 2020;26(31):3847-3861. doi: 10.2174/1381612826666200707131500. Curr Pharm Des. 2020. PMID: 32634076 Review.
-
Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review.Foods. 2021 Jul 9;10(7):1595. doi: 10.3390/foods10071595. Foods. 2021. PMID: 34359469 Free PMC article. Review.
Cited by
-
Development and Characterization of Microparticles with Actinidia arguta Leaves Extract by Spray-Drying: A New Mind-Set Regarding Healthy Compounds for Oral Mucositis.Antioxidants (Basel). 2023 Jul 26;12(8):1496. doi: 10.3390/antiox12081496. Antioxidants (Basel). 2023. PMID: 37627491 Free PMC article.
-
Potential Antidiabetic Activity of Apis mellifera Propolis Extraction Obtained with Ultrasound.Foods. 2024 Jan 22;13(2):348. doi: 10.3390/foods13020348. Foods. 2024. PMID: 38275714 Free PMC article.
-
Generation of Highly Antioxidant Submicron Particles from Myrtus communis Leaf Extract by Supercritical Antisolvent Extraction Process.Antioxidants (Basel). 2023 Feb 20;12(2):530. doi: 10.3390/antiox12020530. Antioxidants (Basel). 2023. PMID: 36830088 Free PMC article.
References
-
- Agarwal R., Tandon P., Gupta V.D. Phonon dispersion in poly (dimethylsilane) Journal of Organometallic Chemistry. 2006;691(13):2902–2908.
-
- Alminger M., Aura A.M., Bohn T., Dufour C., El S.N., Gomes A., et al. In vitro models for studying secondary plant metabolite digestion and bio accessibility. Comprehensive Reviews in Food Science and Food Safety. 2014;13(4):413–436. - PubMed
-
- Amado R.J., Rondón L.P., Prada A.L., Arranz J.C.E., Colarte A.I. Formulación de cápsulas duras de Tamarindus indica L. Revista Cubana de Farmácia. 2014;48(1):118.
-
- Association of Official Analitical Chemistry- A.O.A.C. Official Methods of Analysis of the Association of Analytical Chemistry International. 19th Edition, 2012.
-
- Araújo R.R., Teixeira C.C.C., Freitas L.A.P. The preparation of ternary solid dispersions of an herbal drug via spray drying of liquid feed. Drying Technology. 2010;28(3):412–421.
LinkOut - more resources
Full Text Sources

