Downregulation of protein kinase C gamma reduces epithelial property and enhances malignant phenotypes in colorectal cancer cells
- PMID: 36404918
- PMCID: PMC9672935
- DOI: 10.1016/j.isci.2022.105501
Downregulation of protein kinase C gamma reduces epithelial property and enhances malignant phenotypes in colorectal cancer cells
Abstract
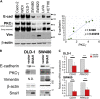
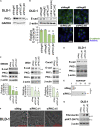
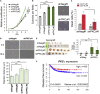
Loss of epithelial integrity is associated with colorectal cancer (CRC) aggressiveness. Protein kinase C (PKC) is frequently implicated in human cancers, but the role of PKCγ in CRC remains poorly understood. Here, we show that PKCγ, a conventional PKC, is expressed in normal colonic epithelium, but this is lower in dedifferentiated CRC. PKCγ expression was downregulated by SNAI1 overexpression, and low PKCγ expression was associated with poor prognosis in patients with CRC. Transient or stable knockdown of PKCγ reduced E-cadherin expression in CRC cells. PKCγ knockdown enhanced proliferation, anchorage-independent cell growth, resistance to anti-cancer drugs, and in vivo tumor growth of DLD-1 cells. We have also identified phosphorylation substrates for PKCγ. Among them, ARHGEF18, a RhoA activator that stabilizes cell-cell junctions, was phosphorylated and stabilized by PKCγ. Thus, these results suggest that the downregulation of PKCγ decreases the epithelial property of CRC cells and enhances its malignant phenotypes.
Keywords: Cancer; Molecular biology.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Immortalized mammary epithelial cells overexpressing protein kinase C gamma acquire a malignant phenotype and become tumorigenic in vivo.Mol Cancer Res. 2003 Aug;1(10):776-87. Mol Cancer Res. 2003. PMID: 12939403
-
Effect of protein kinase Cgamma on gap junction disassembly in lens epithelial cells and retinal cells in culture.Mol Vis. 2002 Mar 14;8:59-66. Mol Vis. 2002. PMID: 11951087
-
Two Sides of the Same Coin: Protein Kinase C γ in Cancer and Neurodegeneration.Front Cell Dev Biol. 2022 Jun 21;10:929510. doi: 10.3389/fcell.2022.929510. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35800893 Free PMC article. Review.
-
Oxidative activation of protein kinase Cgamma through the C1 domain. Effects on gap junctions.J Biol Chem. 2005 Apr 8;280(14):13682-93. doi: 10.1074/jbc.M407762200. Epub 2005 Jan 10. J Biol Chem. 2005. PMID: 15642736
-
Protein Kinase C in the Cerebellum: Its Significance and Remaining Conundrums.Cerebellum. 2018 Feb;17(1):23-27. doi: 10.1007/s12311-017-0898-x. Cerebellum. 2018. PMID: 29134360 Review.
Cited by
-
Genetic Variants at PRKCG Splice and UTR Sites Promote Cancer Susceptibility by Disrupting Epigenetic and miRNA Regulatory Network.J Cancer. 2024 Oct 28;15(20):6644-6657. doi: 10.7150/jca.100911. eCollection 2024. J Cancer. 2024. PMID: 39668814 Free PMC article.
-
Zic family member 5 promotes RIO kinase 3 expression to enhance pancreatic cancer survival.FEBS J. 2025 Aug;292(15):4057-4068. doi: 10.1111/febs.70125. Epub 2025 May 3. FEBS J. 2025. PMID: 40318167 Free PMC article.
-
Curcumin-Induced Molecular Mechanisms in U-87 MG Glioblastoma Cells: Insights from Global Gene Expression Profiling.Molecules. 2025 May 9;30(10):2108. doi: 10.3390/molecules30102108. Molecules. 2025. PMID: 40430278 Free PMC article.
-
Selective chemical and genetic inhibition of PKCβII strongly sensitizes colon cancer cells to anoikis.Mol Biol Rep. 2025 Jun 3;52(1):534. doi: 10.1007/s11033-025-10612-1. Mol Biol Rep. 2025. PMID: 40459703
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

