Non-target impacts of fungicide disturbance on phyllosphere yeasts in conventional and no-till management
- PMID: 36404932
- PMCID: PMC9674006
- DOI: 10.1038/s43705-022-00103-w
Non-target impacts of fungicide disturbance on phyllosphere yeasts in conventional and no-till management
Abstract
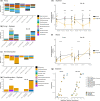
Fungicides reduce fungal pathogen populations and are essential to food security. Understanding the impacts of fungicides on crop microbiomes is vital to minimizing unintended consequences while maintaining their use for plant protection. However, fungicide disturbance of plant microbiomes has received limited attention, and has not been examined in different agricultural management systems. We used amplicon sequencing of fungi and prokaryotes in maize and soybean microbiomes before and after foliar fungicide application in leaves and roots from plots under long-term no-till and conventional tillage management. We examined fungicide disturbance and resilience, which revealed consistent non-target effects and greater resiliency under no-till management. Fungicides lowered pathogen abundance in maize and soybean and decreased the abundance of Tremellomycetes yeasts, especially Bulleribasidiaceae, including core microbiome members. Fungicide application reduced network complexity in the soybean phyllosphere, which revealed altered co-occurrence patterns between yeast species of Bulleribasidiaceae, and Sphingomonas and Hymenobacter in fungicide treated plots. Results indicate that foliar fungicides lower pathogen and non-target fungal abundance and may impact prokaryotes indirectly. Treatment effects were confined to the phyllosphere and did not impact belowground microbial communities. Overall, these results demonstrate the resilience of no-till management to fungicide disturbance, a potential novel ecosystem service provided by no-till agriculture.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effects of synthetic and environmentally friendly fungicides on powdery mildew management and the phyllosphere microbiome of cucumber.PLoS One. 2023 Mar 8;18(3):e0282809. doi: 10.1371/journal.pone.0282809. eCollection 2023. PLoS One. 2023. PMID: 36888572 Free PMC article.
-
Crop Management Impacts the Soybean (Glycine max) Microbiome.Front Microbiol. 2020 Jun 3;11:1116. doi: 10.3389/fmicb.2020.01116. eCollection 2020. Front Microbiol. 2020. PMID: 32582080 Free PMC article.
-
Fungicide effects on fungal community composition in the wheat phyllosphere.PLoS One. 2014 Nov 4;9(11):e111786. doi: 10.1371/journal.pone.0111786. eCollection 2014. PLoS One. 2014. PMID: 25369054 Free PMC article.
-
Evolving challenges and strategies for fungal control in the food supply chain.Fungal Biol Rev. 2021 Jun;36:15-26. doi: 10.1016/j.fbr.2021.01.003. Fungal Biol Rev. 2021. PMID: 34084209 Free PMC article. Review.
-
Harnessing agricultural microbiomes for human pathogen control.ISME Commun. 2022 May 19;2(1):44. doi: 10.1038/s43705-022-00127-2. ISME Commun. 2022. PMID: 37938739 Free PMC article. Review. No abstract available.
Cited by
-
Soil and Phytomicrobiome for Plant Disease Suppression and Management under Climate Change: A Review.Plants (Basel). 2023 Jul 23;12(14):2736. doi: 10.3390/plants12142736. Plants (Basel). 2023. PMID: 37514350 Free PMC article. Review.
-
Agricultural practices and pollinators modulate the anthosphere microbiome.ISME Commun. 2025 Feb 12;5(1):ycaf026. doi: 10.1093/ismeco/ycaf026. eCollection 2025 Jan. ISME Commun. 2025. PMID: 40438189 Free PMC article.
-
Mycobiome of low maintenance iconic landscape plant boxwood under repeated treatments of contact and systemic fungicides.Sci Rep. 2025 Aug 17;15(1):30150. doi: 10.1038/s41598-025-07593-3. Sci Rep. 2025. PMID: 40820086 Free PMC article.
-
Fungicide use intensity influences the soil microbiome and links to fungal disease suppressiveness in amenity turfgrass.Appl Environ Microbiol. 2025 Mar 19;91(3):e0177124. doi: 10.1128/aem.01771-24. Epub 2025 Feb 21. Appl Environ Microbiol. 2025. PMID: 39982054 Free PMC article.
-
Beyond correlation: Understanding the causal link between microbiome and plant health.Heliyon. 2024 Nov 19;10(23):e40517. doi: 10.1016/j.heliyon.2024.e40517. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39669148 Free PMC article. Review.
References
-
- Rykiel EJ. Towards a definition of ecological disturbance. Austral Ecology. 1985;10:361–5. doi: 10.1111/j.1442-9993.1985.tb00897.x. - DOI
-
- Sullivan TP, Sullivan DS. Vegetation management and ecosystem disturbance: impact of glyphosate herbicide on plant and animal diversity in terrestrial systems. Environ Rev. 2003;11:37–59. doi: 10.1139/a03-005. - DOI

