New multienzymatic complex formed between human cathepsin D and snake venom phospholipase A2
- PMID: 36404954
- PMCID: PMC9647731
- DOI: 10.1590/1678-9199-JVATITD-2022-0002
New multienzymatic complex formed between human cathepsin D and snake venom phospholipase A2
Abstract
Background: Cathepsin D (CatD) is a lysosomal proteolytic enzyme expressed in almost all tissues and organs. This protease is a multifunctional enzyme responsible for essential biological processes such as cell cycle regulation, differentiation, migration, tissue remodeling, neuronal growth, ovulation, and apoptosis. The overexpression and hypersecretion of CatD have been correlated with cancer aggressiveness and tumor progression, stimulating cancer cell proliferation, fibroblast growth, and angiogenesis. In addition, some studies report its participation in neurodegenerative diseases and inflammatory processes. In this regard, the search for new inhibitors from natural products could be an alternative against the harmful effects of this enzyme.
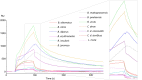
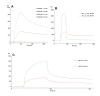
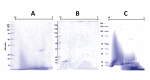
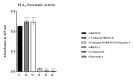
Methods: An investigation was carried out to analyze CatD interaction with snake venom toxins in an attempt to find inhibitory molecules. Interestingly, human CatD shows the ability to bind strongly to snake venom phospholipases A2 (svPLA2), forming a stable muti-enzymatic complex that maintains the catalytic activity of both CatD and PLA2. In addition, this complex remains active even under exposure to the specific inhibitor pepstatin A. Furthermore, the complex formation between CatD and svPLA2 was evidenced by surface plasmon resonance (SPR), two-dimensional electrophoresis, enzymatic assays, and extensive molecular docking and dynamics techniques.
Conclusion: The present study suggests the versatility of human CatD and svPLA2, showing that these enzymes can form a fully functional new enzymatic complex.
Keywords: Cathepsin D; Enzyme complex; Phospholipases A2; Snake venom.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures





Similar articles
-
Beyond Fang's fury: a computational study of the enzyme-membrane interaction and catalytic pathway of the snake venom phospholipase A2 toxin.Chem Sci. 2025 Jan 2;16(4):1974-1985. doi: 10.1039/d4sc06511e. eCollection 2025 Jan 22. Chem Sci. 2025. PMID: 39759936 Free PMC article.
-
Inhibition of enzymatic activities of Bothrops asper snake venom and docking analysis of compounds from plants used in Central America to treat snakebite envenoming.J Ethnopharmacol. 2022 Jan 30;283:114710. doi: 10.1016/j.jep.2021.114710. Epub 2021 Oct 6. J Ethnopharmacol. 2022. PMID: 34626780
-
Characterization of a human coagulation factor Xa-binding site on Viperidae snake venom phospholipases A2 by affinity binding studies and molecular bioinformatics.BMC Struct Biol. 2007 Dec 6;7:82. doi: 10.1186/1472-6807-7-82. BMC Struct Biol. 2007. PMID: 18062812 Free PMC article.
-
Snake venom phospholipase A2 inhibitors: medicinal chemistry and therapeutic potential.Curr Top Med Chem. 2007;7(8):743-56. doi: 10.2174/156802607780487614. Curr Top Med Chem. 2007. PMID: 17456038 Review.
-
Snake venom derived molecules in tumor angiogenesis and its application in cancer therapy; an overview.Curr Top Med Chem. 2015;15(7):649-57. doi: 10.2174/1568026615666150225113402. Curr Top Med Chem. 2015. PMID: 25714377 Review.
References
LinkOut - more resources
Full Text Sources
