Inhibition of Angiogenesis by MiR-524-5p through Suppression of AKT and ERK Activation by Targeting CXCR7 in Colon Cancer Cells
- PMID: 36405246
- PMCID: PMC9671741
- DOI: 10.1155/2022/7224840
Inhibition of Angiogenesis by MiR-524-5p through Suppression of AKT and ERK Activation by Targeting CXCR7 in Colon Cancer Cells
Abstract
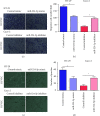
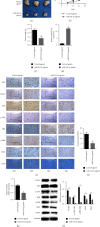
Increasing evidence shows that alterations in microRNA (miRNA) expression are involved in the occurrence and development of various malignant tumors, including colon cancer. MiRNA-524-5p has been reported to have anticancer activity in colon cancer. This study explored the influence of the miRNA-524-5p/CXCR7 axis on angiogenesis using colon cancer cells and further studied the mechanisms involved. We found that changing the expression of miRNA-524-5p can affect colonic proliferation, migration, and angiogenesis. Furthermore, angiogenesis induced by miRNA-524-5p overexpression was reversed by overexpression of CXCR7 in HT-29 cells, while the opposite was observed in Caco-2 cells. Furthermore, miRNA-524-5p inhibited the activation of AKT and ERK signaling by targeting CXCR7. Overall, our results indicated that the miRNA-524-5p/CXCR7 axis regulated angiogenesis in colon cancer cells through the AKT and ERK pathways.
Copyright © 2022 Xiang Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
miR-524-5p inhibits angiogenesis through targeting WNK1 in colon cancer cells.Am J Physiol Gastrointest Liver Physiol. 2020 Apr 1;318(4):G827-G839. doi: 10.1152/ajpgi.00369.2019. Epub 2020 Mar 16. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32174132
-
Chemokine receptor 7 targets the vascular endothelial growth factor via the AKT/ERK pathway to regulate angiogenesis in colon cancer.Cancer Med. 2019 Sep;8(11):5327-5340. doi: 10.1002/cam4.2426. Epub 2019 Jul 26. Cancer Med. 2019. PMID: 31348616 Free PMC article.
-
CircCCNB1 silencing acting as a miR-106b-5p sponge inhibited GPM6A expression to promote HCC progression by enhancing DYNC1I1 expression and activating the AKT/ERK signaling pathway.Int J Biol Sci. 2022 Jan 1;18(2):637-651. doi: 10.7150/ijbs.66915. eCollection 2022. Int J Biol Sci. 2022. PMID: 35002514 Free PMC article.
-
MiR-182-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-C.Cancer Lett. 2020 Sep 28;488:18-26. doi: 10.1016/j.canlet.2020.04.021. Epub 2020 May 27. Cancer Lett. 2020. PMID: 32473243
-
Overexpression of microRNA-30a-5p inhibits liver cancer cell proliferation and induces apoptosis by targeting MTDH/PTEN/AKT pathway.Tumour Biol. 2016 May;37(5):5885-95. doi: 10.1007/s13277-015-4456-1. Epub 2015 Nov 21. Tumour Biol. 2016. PMID: 26589417
References
LinkOut - more resources
Full Text Sources
Miscellaneous

