Synthesis, characterization, and biological activities of zinc(II), copper(II) and nickel(II) complexes of an aminoquinoline derivative
- PMID: 36405328
- PMCID: PMC9669718
- DOI: 10.3389/fchem.2022.1053532
Synthesis, characterization, and biological activities of zinc(II), copper(II) and nickel(II) complexes of an aminoquinoline derivative
Abstract
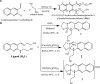
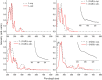
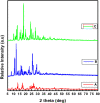
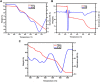
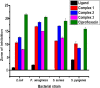
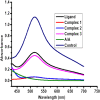
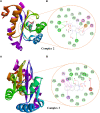
Interest is increasingly focused on the use of transition metal complexes as biochemical, medical, analytical, pharmaceutical, agronomic, anticancer, and antibacterial agents. In this study, three complexes of [Zn(H2L)Cl] (1), [Cu(H2L)(H2O)(NO3)] (2) and [Ni(H2L)(NO3)].2H2O (3) were synthesized from a 2-chloroquinoline-3-carbaldehyde derived ligand [H3L = ((E)-2-(((2-((2-hydroxyethyl)amino)quinolin-3-yl)methylene)amino)ethanol. The compounds were characterized using physicochemical and spectroscopic methods. The results demonstrate that the free ligand behaves as a tridentate ligand with one oxygen and two nitrogen (ONN) donor atoms in 1:1 metal:ligand ratio. The formation constants of the complexes were found to be (K Zn(II) = 2.3 × 106, K Cu(II) = 2.9 × 106, and K Ni(II) = 3.8 × 105). The thermodynamic parameters indicated that the reactions were spontaneous with exothermic nature of metal-ligand interaction energies. Based on the analyses of the experimental (EDX, FTIR, PXRD, MS and TGA) and DFT results, a distorted tetrahedral, a distorted square pyramidal and square planar geometry for Zn(II), Cu(II) and Ni(II) complexes, respectively, were proposed. The B3LYP calculated IR frequencies and TD-B3LYP calculated absorption spectra were found to be in good agreement with the corresponding experimental results. The powder XRD data confirmed that the Zn(II), Cu(II) and Ni(II) complexes have polycrystalline nature with average crystallite sizes of 27.86, 33.54, 37.40 Å, respectively. In vitro antibacterial activity analyses of the complexes were studied with disk diffusion method, in which the complexes showed better activity than the precursor ligand. Particularly the Cu(II) complex showed higher percent activity index (62, 90%), than both Zn(II) (54, 82%) and Ni(II) (41, 68%) complexes against both E. coli and P. aeruginosa, respectively. Using the DPPH assay, the complexes were further assessed for their antioxidant capacities. All metal complexes showed improved antioxidant activity than the free ligand. Zn(II) and Cu(II) complexes, which had IC50 values of 10.46 and 8.62 μg/ml, respectively, showed the best antioxidant activity. The calculated results of Lipinski's rule of five also showed that the target complexes have drug-like molecular nature and similarly, the results of binding mode of action of these compounds against E. coli DNA gyrase B and P. aeruginosa LasR.DNA were found to be in good agreement with the in vitro biological activities.
Keywords: DFT analysis; aminoquinoline; antibacterial; antioxidant; molecular docking; novel metal complexes.
Copyright © 2022 Damena, Alem, Zeleke, Desalegn, Eswaramoorthy and Demissie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abane-Merzouk L., Adkhis A., Terrachet-Bouaziz S., Makhloufi-Chebli M. (2019). Synthesis, DFT/TD-DFT theoretical studies, experimental characterization, electrochemical and antioxidant activity of Fe(III) complexes of bis (dimethylglyoximato) guanine. J. Mol. Struct. 1186, 413–422. 10.1016/j.molstruc.2019.02.108 - DOI
-
- Abd El-Halim H. F., Mohamed G. G., Khalil E. A. M. (2017). Synthesis, spectral, thermal and biological studies of mixed ligand complexes with newly prepared Schiff base and 1, 10-phenanthroline ligands. J. Mol. Struct. 1146, 153–163. 10.1016/j.molstruc.2017.05.092 - DOI
-
- Abumelha H. M., Alkhatib F., Alzahrani S., Abualnaja M., Alsaigh S., Alfaifi M. Y., et al. (2021). Synthesis and characterization for pharmaceutical models from Co(II), Ni(II) and Cu(II)-thiophene complexes; apoptosis, various theoretical studies and pharmacophore modeling. J. Mol. Liq. 328, 115483–115512. 10.1016/j.molliq.2021.115483 - DOI
-
- Ahmad S., Hamid F., Hamid M., Sarwar S., Memon A. N., Ghangro A. B. (2012). Spectrophotometric study of stability constants of cimetidine – Ni ( II ) complex at different temperatures. Arab. J. Chem. 5, 309–314. 10.1016/j.arabjc.2010.09.009 - DOI
LinkOut - more resources
Full Text Sources

