Real-life effectiveness of transitioning from paliperidone palmitate 1-monthly to paliperidone palmitate 3-monthly long-acting injectable formulation
- PMID: 36405400
- PMCID: PMC9666838
- DOI: 10.1177/20451253221136021
Real-life effectiveness of transitioning from paliperidone palmitate 1-monthly to paliperidone palmitate 3-monthly long-acting injectable formulation
Abstract
Background: Non-adherence to antipsychotics in schizophrenia is associated with an increased risk of psychotic relapse and hospitalization, a risk that is reduced with the use of long-acting injectable (LAI) antipsychotics. Randomized clinical trials (RCTs) have demonstrated the efficacy of paliperidone palmitate 3-monthly (PP3M) for psychotic relapse prevention in schizophrenia, but it remains poorly documented among individuals treated in real-life settings who can benefit the most out of LAIs.
Objectives: The objective of this study was to evaluate the effectiveness of PP3M in relapse prevention among patients with schizophrenia.
Methods: This is a multicentre retrospective study conducted in four outpatients' clinics across Canada. All consecutive patients with a main diagnosis of schizophrenia who initiated PP3M between June 2016 and March 2020 were included. The primary outcome was psychotic relapse, defined using broad and clinically relevant criteria.
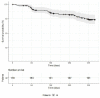
Results: Among 178 consecutive patients who were switched to PP3M, the 12-month relapse rate was 18.5% and the relapse-free survival probability was 0.788 (95% confidence interval [CI] = 0.725-0.856). Comorbid diagnoses of personality disorders and substance use disorders were associated with hazard rates (HRs) of 3.6 (95% CI = 1.8-7.3, p < 0.001) and 3.1 (95% CI = 1.6-6.2), respectively. Increased psychopathology severity was associated with an increased likelihood of relapse, while having a job or being in school was protective.
Conclusion: These findings reinforce the necessity of conducting research in patients with comorbid psychiatric disorders who are typically underrepresented in RCTs, yet overrepresented in real-life settings, in order to better inform and guide clinical practice.
Keywords: antipsychotic; long-acting injectable; naturalistic setting; paliperidone palmitate 1-monthly; paliperidone palmitate 3-monthly; psychotic disorder; relapse; schizophrenia.
© The Author(s), 2022.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: OC has received honorariums from AbbVie, Janssen Canada and Otsuka-Lundbeck Alliance. MFD has received grants from AbbVie, Janssen Canada and Otsuka-Lundbeck Alliance. MAR has received grants from AbbVie, Janssen Canada, Mylan, Otsuka-Lundbeck Alliance, and Sunovion. All other authors have no conflicts of interest that are directly relevant to the content of this study.
Figures
References
-
- Brodeur S, Vanasse A, Courteau J, et al.. Comparative effectiveness and safety of antipsychotic drugs in patients with schizophrenia initiating or reinitiating treatment: a real-world observational study. Acta Psychiatr Scand 2022; 145: 456–468. - PubMed
-
- Greene M, Yan T, Chang E, et al.. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ 2018; 21: 127–134. - PubMed
-
- Kishimoto T, Hagi K, Kurokawa S, et al.. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 2021; 8: 387–404. - PubMed
-
- Berwaerts J, Liu Y, Gopal S, et al.. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry 2015; 72: 830–839. - PubMed
LinkOut - more resources
Full Text Sources