Using Gene Expression Profiling to Personalize Skin Cancer Management
- PMID: 36405422
- PMCID: PMC9664966
Using Gene Expression Profiling to Personalize Skin Cancer Management
Abstract
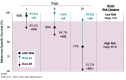
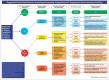
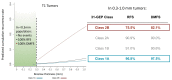
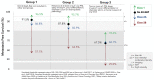
Risk-stratification of cancer, traditionally performed through staging, directs optimal disease management decisions with the result of improved patient outcomes. Many forms of cutaneous cancer have overall excellent survival rates, but conventional staging methods are imperfect in identifying high-risk patients. Gene expression profiling (GEP) is a clinically available, objective metric that can be used in conjunction with traditional clinicopathological staging to help clinicians stratify risk in patients with skin cancer, even in those who lack traditional risk markers. For patients with melanoma, the 31-GEP test provides personalized prognostic information that can guide risk-appropriate clinical management and surveillance decisions. The i31-GEP integrates 31-GEP results with clinicopathological features to provide a risk of recurrence (i31-GEP for ROR) and likelihood of having a positive sentinel lymph node biopsy (SLNB) (i31-GEP for SLNB) for patients with melanoma. For patients with cutaneous squamous cell carcinoma who have at least one risk factor, the 40-GEP test allows for better risk stratification by identifying the high-risk patients who are most likely to develop metastasis. These tests can be easily integrated into clinical practice to help guide treatment choices.
Keywords: 31-GEP; 40-GEP; cutaneous squamous cell carcinoma; gene expression profiling; i31-GEP; melanoma; prognosis; risk of recurrence; risk stratification; sentinel lymph node biopsy.
Copyright © 2022. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Ms. LeQuang reports no conflicts of interest relevant to the content of this article.
Figures








References
-
- https://www.cancer.gov/news-events/cancer-currents-blog/2020/metastatic-... NCI staff. Deaths from metastatic melanoma drop substantially in the United States. 21 Apr 2020. National Institute of Health. National Cancer Institute. Accessed 13 Jun 2022.
-
- Hodi FS, Chiarion-Sileni V, Gonzalez R et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
