Repeated porphyrin lipoprotein-based photodynamic therapy controls distant disease in mouse mesothelioma via the abscopal effect
- PMID: 36405502
- PMCID: PMC9646247
- DOI: 10.1515/nanoph-2021-0241
Repeated porphyrin lipoprotein-based photodynamic therapy controls distant disease in mouse mesothelioma via the abscopal effect
Abstract
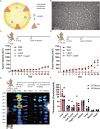
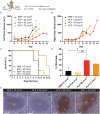
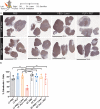
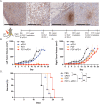
While photodynamic therapy (PDT) can induce acute inflammation in the irradiated tumor site, a sustained systemic, adaptive immune response is desirable, as it may control the growth of nonirradiated distant disease. Previously, we developed porphyrin lipoprotein (PLP), a ∼20 nm nanoparticle photosensitizer, and observed that it not only efficiently eradicated irradiated primary VX2 buccal carcinomas in rabbits, but also induced regression of nonirradiated metastases in a draining lymph node. We hypothesized that PLP-mediated PDT can induce an abscopal effect and we sought to investigate the immune mechanism underlying such a response in a highly aggressive, dual subcutaneous AE17-OVA+ mesothelioma model in C57BL/6 mice. Four cycles of PLP-mediated PDT was sufficient to delay the growth of a distal, nonirradiated tumor four-fold relative to controls. Serum cytokine analysis revealed high interleukin-6 levels, showing a 30-fold increase relative to phosphate-buffered solution (PBS) treated mice. Flow cytometry revealed an increase in CD4+ T cells and effector memory CD8+ T cells in non-irradiated tumors. Notably, PDT in combination with PD-1 antibody therapy prolonged survival compared to monotherapy and PBS. PLP-mediated PDT shows promise in generating a systemic immune response that can complement other treatments, improving prognoses for patients with metastatic cancers.
Keywords: PD-1; immune response; immunotherapy; photodynamic therapy; porphyrin; thoracic malignant tumor.
© 2021 Jenny Lou et al., published by De Gruyter, Berlin/Boston.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
