Optimized Mouse-on-mouse Immunohistochemical Detection of Mouse ESR2 Proteins with PPZ0506 Monoclonal Antibody
- PMID: 36405553
- PMCID: PMC9631985
- DOI: 10.1267/ahc.22-00043
Optimized Mouse-on-mouse Immunohistochemical Detection of Mouse ESR2 Proteins with PPZ0506 Monoclonal Antibody
Abstract
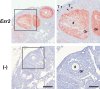
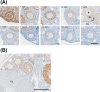
Despite the physiological significance of ESR2, a lack of well-validated detection systems for ESR2 proteins has hindered progress in ESR2 research. Thus, recent identification of a specific anti-human ESR2 monoclonal antibody (PPZ0506) and its specific cross-reactivity against mouse and rat ESR2 proteins heightened momenta toward development of appropriate immunohistochemical detection systems for rodent ESR2 proteins. Building upon our previous optimization of ESR2 immunohistochemical detection in rats using PPZ0506, in this study, we further aimed to optimize mouse-on-mouse immunohistochemical detection using PPZ0506. Our assessment of several staining conditions using paraffin-embedded ovary sections revealed that intense heat-induced antigen retrieval, appropriate blocking, and appropriate antibody dilutions were necessary for optimization of mouse-on-mouse immunohistochemistry. Subsequently, we applied the optimized immunostaining method to determine expression profiles of mouse ESR2 proteins in peripheral tissues and brain subregions. Our analyses revealed more localized distribution of mouse ESR2 proteins than previously assumed. Moreover, comparison of these results with those obtained in humans and rats using PPZ0506 revealed interspecies differences in ESR2 expression. We expect that our optimized methodology for immunohistochemical staining of mouse ESR2 proteins will help researchers to solve multiple lines of controversial evidence concerning ESR2 expression.
Keywords: ERβ; ESR2; PPZ0506; estrogen receptor β; immunohistochemistry.
2022 The Japan Society of Histochemistry and Cytochemistry.
Conflict of interest statement
VThe authors declare that they have no conflicts of interest to declare.
Figures





References
-
- Antonson, P., Apolinário, L. M., Shamekh, M. M., Humire, P., Poutanen, M., Ohlsson, C., et al. (2020) Generation of an all-exon Esr2 deleted mouse line: Effects on fertility. Biochem. Biophys. Res. Commun. 529; 231–237. - PubMed
-
- Couse, J. F., Lindzey, J., Grandien, K., Gustafsson, J. A. and Korach, K. S. (1997) Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138; 4613–4621. - PubMed

