Primary antibody response after influenza virus infection is first dominated by low-mutated HA-stem antibodies followed by higher-mutated HA-head antibodies
- PMID: 36405682
- PMCID: PMC9670313
- DOI: 10.3389/fimmu.2022.1026951
Primary antibody response after influenza virus infection is first dominated by low-mutated HA-stem antibodies followed by higher-mutated HA-head antibodies
Abstract
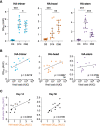
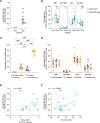
Several studies have shown that the first encounter with influenza virus shapes the immune response to future infections or vaccinations. However, a detailed analysis of the primary antibody response is lacking as this is difficult to study in humans. It is therefore not known what the frequency and dynamics of the strain-specific hemagglutinin (HA) head- and stem-directed antibody responses are directly after primary influenza virus infection. Here, sera of twelve H1N1pdm2009 influenza virus-infected cynomolgus macaques were evaluated for HA-head and HA-stem domain antibody responses. We observed an early induction of HA-stem antibody responses, which was already decreased by day 56. In contrast, responses against the HA-head domain were low early after infection and increased at later timepoint. The HA-specific B cell repertoires in each animal showed diverse VH-gene usage with preferred VH-gene and JH-gene family usage for HA-head or HA-stem B cells but a highly diverse allelic variation within the VH-usage. HA-head B cells had shorter CDRH3s and higher VH-gene somatic hyper mutation levels relative to HA-stem B cells. In conclusion, our data suggest that HA-stem antibodies are the first to react to the infection while HA-head antibodies show a delayed response, but a greater propensity to enter the germinal center and undergo affinity maturation.
Keywords: HA-head; HA-stem; antibody response; hemagglutinin; influenza A virus; primary response.
Copyright © 2022 Aartse, Mortier, Mooij, Hofman, van Haaren, Corcoran, Karlsson Hedestam, Eggink, Claireaux, Bogers, van Gils and Koopman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

