Leishmania kinetoplast DNA contributes to parasite burden in infected macrophages: Critical role of the cGAS-STING-TBK1 signaling pathway in macrophage parasitemia
- PMID: 36405710
- PMCID: PMC9667060
- DOI: 10.3389/fimmu.2022.1007070
Leishmania kinetoplast DNA contributes to parasite burden in infected macrophages: Critical role of the cGAS-STING-TBK1 signaling pathway in macrophage parasitemia
Abstract
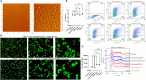
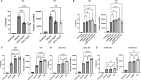
Leishmania parasites harbor a unique network of circular DNA known as kinetoplast DNA (kDNA). The role of kDNA in leishmania infections is poorly understood. Herein, we show that kDNA delivery to the cytosol of Leishmania major infected THP-1 macrophages provoked increased parasite loads when compared to untreated cells, hinting at the involvement of cytosolic DNA sensors in facilitating parasite evasion from the immune system. Parasite proliferation was significantly hindered in cGAS- STING- and TBK-1 knockout THP-1 macrophages when compared to wild type cells. Nanostring nCounter gene expression analysis on L. major infected wild type versus knockout cells revealed that some of the most upregulated genes including, Granulysin (GNLY), Chitotriosidase-1 (CHIT1), Sialomucin core protein 24 (CD164), SLAM Family Member 7 (SLAMF7), insulin-like growth factor receptor 2 (IGF2R) and apolipoprotein E (APOE) were identical in infected cGAS and TBK1 knockout cells, implying their involvement in parasite control. Amlexanox treatment (a TBK1 inhibitor) of L. major infected wild type cells inhibited both the percentage and the parasite load of infected THP-1 cells and delayed footpad swelling in parasite infected mice. Collectively, these results suggest that leishmania parasites might hijack the cGAS-STING-TBK1 signaling pathway to their own advantage and the TBK1 inhibitor amlexanox could be of interest as a candidate drug in treatment of cutaneous leishmaniasis.
Keywords: 2'3'-cGAMP; H151; STING; TBK1; amlexanox; cGAS; kinetoplast DNA (kDNA); leishmania.
Copyright © 2022 Yilmaz, Dunuroglu, Ayanoglu, Ipekoglu, Yildirim, Girginkardesler, Ozbel, Toz, Ozbilgin, Aykut, Gursel and Gursel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The cGas-Sting Signaling Pathway Is Required for the Innate Immune Response Against Ectromelia Virus.Front Immunol. 2018 Jun 14;9:1297. doi: 10.3389/fimmu.2018.01297. eCollection 2018. Front Immunol. 2018. PMID: 29963044 Free PMC article.
-
African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway.J Virol. 2019 May 29;93(12):e02298-18. doi: 10.1128/JVI.02298-18. Print 2019 Jun 15. J Virol. 2019. PMID: 30918080 Free PMC article.
-
cGAS exacerbates Schistosoma japonicum infection in a STING-type I IFN-dependent and independent manner.PLoS Pathog. 2022 Feb 2;18(2):e1010233. doi: 10.1371/journal.ppat.1010233. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35108342 Free PMC article.
-
Inhibitory targeting cGAS-STING-TBK1 axis: Emerging strategies for autoimmune diseases therapy.Front Immunol. 2022 Sep 12;13:954129. doi: 10.3389/fimmu.2022.954129. eCollection 2022. Front Immunol. 2022. PMID: 36172373 Free PMC article. Review.
-
STING or Sting: cGAS-STING-Mediated Immune Response to Protozoan Parasites.Trends Parasitol. 2020 Sep;36(9):773-784. doi: 10.1016/j.pt.2020.07.001. Epub 2020 Jul 28. Trends Parasitol. 2020. PMID: 32736985 Review.
Cited by
-
Memory T cells: promising biomarkers for evaluating protection and vaccine efficacy against leishmaniasis.Front Immunol. 2024 Feb 26;15:1304696. doi: 10.3389/fimmu.2024.1304696. eCollection 2024. Front Immunol. 2024. PMID: 38469319 Free PMC article. Review.
-
Aflatoxin B1 Induced Oxidative Stress and Gut Microbiota Disorder to Increase the Infection of Cyprinid Herpesvirus 2 in Gibel Carp (Carassius auratus gibelio).Antioxidants (Basel). 2023 Jan 28;12(2):306. doi: 10.3390/antiox12020306. Antioxidants (Basel). 2023. PMID: 36829867 Free PMC article.
-
cGAS-STING Pathway Activation during Trypanosoma cruzi Infection Leads to Tissue-Dependent Parasite Control.J Immunol. 2023 Oct 1;211(7):1123-1133. doi: 10.4049/jimmunol.2300373. J Immunol. 2023. PMID: 37603014 Free PMC article.
-
cGAS-STING signaling pathway in intestinal homeostasis and diseases.Front Immunol. 2023 Sep 14;14:1239142. doi: 10.3389/fimmu.2023.1239142. eCollection 2023. Front Immunol. 2023. PMID: 37781354 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

