Anti-inflammatory mechanisms and pharmacological actions of phycocyanobilin in a mouse model of experimental autoimmune encephalomyelitis: A therapeutic promise for multiple sclerosis
- PMID: 36405721
- PMCID: PMC9669316
- DOI: 10.3389/fimmu.2022.1036200
Anti-inflammatory mechanisms and pharmacological actions of phycocyanobilin in a mouse model of experimental autoimmune encephalomyelitis: A therapeutic promise for multiple sclerosis
Abstract
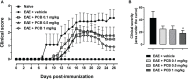
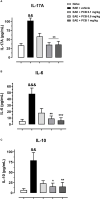
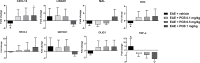
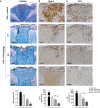
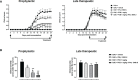
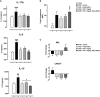
Cytokines, demyelination and neuroaxonal degeneration in the central nervous system are pivotal elements implicated in the pathogenesis of multiple sclerosis (MS) and its nonclinical model of experimental autoimmune encephalomyelitis (EAE). Phycocyanobilin (PCB), a chromophore of the biliprotein C-Phycocyanin (C-PC) from Spirulina platensis, has antioxidant, immunoregulatory and anti-inflammatory effects in this disease, and it could complement the effect of other Disease Modifying Treatments (DMT), such as Interferon-β (IFN-β). Here, our main goal was to evaluate the potential PCB benefits and its mechanisms of action to counteract the chronic EAE in mice. MOG35-55-induced EAE was implemented in C57BL/6 female mice. Clinical signs, pro-inflammatory cytokines levels by ELISA, qPCR in the brain and immunohistochemistry using precursor/mature oligodendrocytes cells antibodies in the spinal cord, were assessed. PCB enhanced the neurological condition, and waned the brain concentrations of IL-17A and IL-6, pro-inflammatory cytokines, in a dose-dependent manner. A down- or up-regulating activity of PCB at 1 mg/kg was identified in the brain on three (LINGO1, NOTCH1, and TNF-α), and five genes (MAL, CXCL12, MOG, OLIG1, and NKX2-2), respectively. Interestingly, a reduction of demyelination, active microglia/macrophages density, and axonal damage was detected along with an increase in oligodendrocyte precursor cells and mature oligodendrocytes, when assessed the spinal cords of EAE mice that took up PCB. The studies in vitro in rodent encephalitogenic T cells and in vivo in the EAE mouse model with the PCB/IFN-β combination, showed an enhanced positive effect of this combined therapy. Overall, these results demonstrate the anti-inflammatory activity and the protective properties of PCB on the myelin and support its use with IFN-β as an improved DMT combination for MS.
Keywords: antioxidants; experimental autoimmune encephalomyelitis; interferon-β; multiple sclerosis; phycocyanobilin; proinflammatory cytokines; remyelination.
Copyright © 2022 Marín-Prida, Pavón-Fuentes, Lagumersindez-Denis, Camacho-Rodríguez, García-Soca, Sarduy-Chávez, Vieira, Carvalho-Tavares, Falcón-Cama, Fernández-Massó, Hernández-González, Martínez-Donato, Guillén-Nieto, Pentón-Arias, Teixeira and Pentón-Rol.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Beneficial effects of oral administration of C-Phycocyanin and Phycocyanobilin in rodent models of experimental autoimmune encephalomyelitis.Life Sci. 2018 Feb 1;194:130-138. doi: 10.1016/j.lfs.2017.12.032. Epub 2017 Dec 27. Life Sci. 2018. PMID: 29287781
-
Comparative Neuroregenerative Effects of C-Phycocyanin and IFN-Beta in a Model of Multiple Sclerosis in Mice.J Neuroimmune Pharmacol. 2016 Mar;11(1):153-67. doi: 10.1007/s11481-015-9642-9. Epub 2015 Nov 10. J Neuroimmune Pharmacol. 2016. PMID: 26556034
-
Nobiletin attenuates inflammation via modulating proinflammatory and antiinflammatory cytokine expressions in an autoimmune encephalomyelitis mouse model.Fitoterapia. 2022 Jan;156:105099. doi: 10.1016/j.fitote.2021.105099. Epub 2021 Dec 9. Fitoterapia. 2022. PMID: 34896483
-
C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective.Behav Sci (Basel). 2018 Jan 18;8(1):15. doi: 10.3390/bs8010015. Behav Sci (Basel). 2018. PMID: 29346320 Free PMC article. Review.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
-
The effects of Phycocyanobilin on experimental arthritis involve the reduction in nociception and synovial neutrophil infiltration, inhibition of cytokine production, and modulation of the neuronal proteome.Front Immunol. 2023 Oct 23;14:1227268. doi: 10.3389/fimmu.2023.1227268. eCollection 2023. Front Immunol. 2023. PMID: 37936684 Free PMC article.
-
Nutraceutical Features of the Phycobiliprotein C-Phycocyanin: Evidence from Arthrospira platensis (Spirulina).Nutrients. 2024 Jun 3;16(11):1752. doi: 10.3390/nu16111752. Nutrients. 2024. PMID: 38892686 Free PMC article. Review.
-
Antioxidant Therapies in the Treatment of Multiple Sclerosis.Biomolecules. 2024 Oct 8;14(10):1266. doi: 10.3390/biom14101266. Biomolecules. 2024. PMID: 39456199 Free PMC article. Review.
-
Oligodendrocyte precursor cell-derived exosomes combined with cell therapy promote clinical recovery by immunomodulation and gliosis attenuation.Front Cell Neurosci. 2024 Jul 23;18:1413843. doi: 10.3389/fncel.2024.1413843. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39109218 Free PMC article.
-
Effects of spirulina (Arthrospira) platensis supplementation on inflammation, physical and mental quality of life, and anthropometric measures in patients with relapsing-remitting multiple sclerosis (RRMS): a triple-blinded, randomized, placebo-controlled trial.Nutr J. 2025 Aug 28;24(1):132. doi: 10.1186/s12937-025-01200-x. Nutr J. 2025. PMID: 40877830 Free PMC article. Clinical Trial.
References
-
- Pentón-Rol G, Martínez-Sánchez G, Cervantes-Llanos M, Lagumersindez-Denis N, Acosta-Medina EF, Falcón-Cama V, et al. . C-phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int Immunopharmacol (2011) 11(1):29–38. doi: 10.1016/j.intimp.2010.10.001 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

