Low dose versus standard dose rituximab for the treatment of antiphospholipid syndrome: A pilot study from a tertiary medical center
- PMID: 36405743
- PMCID: PMC9670802
- DOI: 10.3389/fimmu.2022.971366
Low dose versus standard dose rituximab for the treatment of antiphospholipid syndrome: A pilot study from a tertiary medical center
Abstract
Background: To investigate the therapeutic effects and safety of low-dose and standard-dose rituximab (RTX) in the treatment of antiphospholipid syndrome (APS).
Methods: In this real-world study, we included 22 consecutive patients with APS who received RTX. Standard dose (SD) was defined as an overall dosage of RTX ≥ 1000mg in the induction period, and low dose (LD) was defined as an overall dosage of RTX <1000mg.
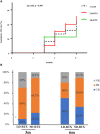
Results: Of included patients, 1 patients died, 2 patients withdrew and 19 patients completed 6-month follow-up. Nine patients received SD-RTX and 13 patients received LD-RTX, and elder patients [LD-RTX vs. SD-RTX: (49.1 ± 15.5) vs. (35.8 ± 12.3) years, p = 0.044] and patients with later-onset [LD-RTX vs. SD-RTX: (46.8 ± 16.3) vs. (31.3 ± 13.6) years, p = 0.029] were more frequently included in LD-RTX than SD-RTX. Following 6 month RTX treatment, 8 patients (42.1%) achieved complete remission, 8 patients (42.1%) achieved partial remission and 3 patients (15.8%) showed no remission. The titers of anticardiolipin antibodies [baseline vs. 6 months: 30.8 (10.7, 90) vs. 19.5 (2.45, 69.10) U/L, p = 0.023] and the levels of erythrocyte sedimentation rate [baseline vs. 6 months: 29 (6, 63) vs. '6 (3, 14) mm/h, p = 0.021] exhibited a significantly decrease in all APS patients. Remission rate and titers of anti-β2-glycoprotein I and lupus anticoagulant did not differ significantly between two groups.
Conclusion: RTX might be a safe and effective option for patients with APS, and low dose confers equal efficacy as standard dose. Further cohort studies are needed to confirm our findings.
Keywords: antiphospholipid syndrome; low dose; real-world study; rituximab; standard dose.
Copyright © 2022 Gan, Zhong, Zhao, Li, Ye and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Rituximab for refractory manifestations of the antiphospholipid syndrome: a multicentre Israeli experience.Clin Exp Rheumatol. 2021 Sep-Oct;39(5):1049-1055. doi: 10.55563/clinexprheumatol/cc5taf. Epub 2020 Oct 27. Clin Exp Rheumatol. 2021. PMID: 33124581
-
Effectiveness and safety of low dose Rituximab as remission-maintenance treatment for patients with refractory idiopathic inflammatory myopathies: results of a retrospective study from a monocentric cohort.Clin Rheumatol. 2024 Oct;43(10):3167-3174. doi: 10.1007/s10067-024-07079-z. Epub 2024 Aug 28. Clin Rheumatol. 2024. PMID: 39196499 Free PMC article.
-
Asthma control in eosinophilic granulomatosis with polyangiitis treated with rituximab.Clin Rheumatol. 2020 May;39(5):1581-1590. doi: 10.1007/s10067-019-04891-w. Epub 2020 Jan 2. Clin Rheumatol. 2020. PMID: 31897956
-
Rituximab in children with steroid-dependent nephrotic syndrome: experience of a tertiary center and review of the literature.Acta Clin Belg. 2017 Jun;72(3):147-155. doi: 10.1080/17843286.2016.1208955. Epub 2016 Jul 13. Acta Clin Belg. 2017. PMID: 27409338 Review.
-
Anti B-cell therapy against refractory thrombocytopenia in SLE and MCTD patients: long-term follow-up and review of the literature.Lupus. 2013 Jun;22(7):664-74. doi: 10.1177/0961203313485489. Epub 2013 Apr 23. Lupus. 2013. PMID: 23612795 Review.
Cited by
-
An update on the biologics for the treatment of antiphospholipid syndrome.Front Immunol. 2023 May 19;14:1145145. doi: 10.3389/fimmu.2023.1145145. eCollection 2023. Front Immunol. 2023. PMID: 37275894 Free PMC article. Review.
References
-
- Rudnicka D, Oszmiana A, Finch DK, Strickland I, Schofield DJ, Lowe DC, et al. . Rituximab causes a polarization of b cells that augments its therapeutic function in NK-cell-mediated antibody-dependent cellular cytotoxicity. Blood (2013) 121(23):4694–702. doi: 10.1182/blood-2013-02-482570 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

