Mesenchymal stromal cell extracellular vesicles for multiple sclerosis in preclinical rodent models: A meta-analysis
- PMID: 36405749
- PMCID: PMC9673165
- DOI: 10.3389/fimmu.2022.972247
Mesenchymal stromal cell extracellular vesicles for multiple sclerosis in preclinical rodent models: A meta-analysis
Abstract
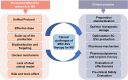
Introduction: Extracellular vesicles (EVs), especially mesenchymal stem (stromal) cell-derived EVs (MSC-EVs), have gained attention as potential novel treatments for multiple sclerosis (MS). However, their effects remain incompletely understood. Thus, the purpose of this meta-analysis was to systematically review the efficacy of MSC-EVs in preclinical rodent models of MS.
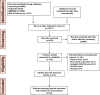
Methods: We searched PubMed, EMBASE, and the Web of Science databases up to August 2021 for studies that reported the treatment effects of MSC-EVs in rodent MS models. The clinical score was extracted as an outcome. Articles were peer-reviewed by two authors based on the inclusion and exclusion criteria. This meta-analysis was conducted using Stata 15.1 and R.
Results: A total of twelve animal studies met the inclusion criteria. In our study, the MSC-EVs had a positive overall effect on the clinical score with a standardized mean difference (SMD) of -2.17 (95% confidence interval (CI)):-3.99 to -0.34, P = 0.01). A significant amount of heterogeneity was observed among the studies.
Conclusions: This meta-analysis suggests that transplantation of MSC-EVs in MS rodent models improved functional recovery. Additionally, we identified several critical knowledge gaps, such as insufficient standardized dosage units and uncertainty regarding the optimal dose of MSC-EVs transplantation in MS. These gaps must be addressed before clinical trials can begin with MSC-EVs.
Keywords: animal model; exosomes; extracellular vesicles; meta-analysis; multiple sclerosis; stem cells; systematic review.
Copyright © 2022 Xun, Deng, Zhao, Ge and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Mesenchymal Stromal Cell-derived Extracellular Vesicles in Preclinical Animal Models of Tumor Growth: Systematic Review and Meta-analysis.Stem Cell Rev Rep. 2022 Mar;18(3):993-1006. doi: 10.1007/s12015-021-10163-5. Epub 2021 Apr 15. Stem Cell Rev Rep. 2022. PMID: 33860455
-
Extracellular vesicles for acute kidney injury in preclinical rodent models: a meta-analysis.Stem Cell Res Ther. 2020 Jan 3;11(1):11. doi: 10.1186/s13287-019-1530-4. Stem Cell Res Ther. 2020. PMID: 31900218 Free PMC article.
-
Therapeutic potential of stem cell extracellular vesicles for ischemic stroke in preclinical rodent models: a meta-analysis.Stem Cell Res Ther. 2023 Apr 3;14(1):62. doi: 10.1186/s13287-023-03270-2. Stem Cell Res Ther. 2023. PMID: 37013588 Free PMC article.
-
MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: a Systematic Review and Meta-Analysis of Preclinical Animal Studies.Stem Cell Rev Rep. 2022 Mar;18(3):968-979. doi: 10.1007/s12015-021-10164-4. Epub 2021 Apr 24. Stem Cell Rev Rep. 2022. PMID: 33893619 Free PMC article.
-
Mesenchymal stem cells-derived therapies for subarachnoid hemorrhage in preclinical rodent models: a meta-analysis.Stem Cell Res Ther. 2022 Jan 29;13(1):42. doi: 10.1186/s13287-022-02725-2. Stem Cell Res Ther. 2022. PMID: 35093176 Free PMC article.
Cited by
-
From bench to bedside: translating mesenchymal stem cell therapies through preclinical and clinical evidence.Front Bioeng Biotechnol. 2025 Jul 30;13:1639439. doi: 10.3389/fbioe.2025.1639439. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40809065 Free PMC article. Review.
-
Stem cell therapies: a new era in the treatment of multiple sclerosis.Front Neurol. 2024 May 9;15:1389697. doi: 10.3389/fneur.2024.1389697. eCollection 2024. Front Neurol. 2024. PMID: 38784908 Free PMC article. Review.
-
Interactions Between Extracellular Vesicles and Autophagy in Neuroimmune Disorders.Neurosci Bull. 2024 Jul;40(7):992-1006. doi: 10.1007/s12264-024-01183-5. Epub 2024 Feb 29. Neurosci Bull. 2024. PMID: 38421513 Free PMC article. Review.
-
Emerging role of extracellular vesicles in multiple sclerosis: From cellular surrogates to pathogenic mediators and beyond.J Neuroimmunol. 2023 Apr 15;377:578064. doi: 10.1016/j.jneuroim.2023.578064. Epub 2023 Mar 11. J Neuroimmunol. 2023. PMID: 36934525 Free PMC article. Review.
-
Extracellular Vesicles in Multiple Sclerosis: Their Significance in the Development and Possible Applications as Therapeutic Agents and Biomarkers.Genes (Basel). 2024 Jun 12;15(6):772. doi: 10.3390/genes15060772. Genes (Basel). 2024. PMID: 38927708 Free PMC article. Review.
References
-
- American Association of Neurological Surgeons. American Society of Neuroradiology. Cardiovascular and Interventional Radiology Society of Europe. Canadian Interventional Radiology Association. Congress of Neurological Surgeons. European Society of Minimally Invasive Neurological Therapy et al. . Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke (2018) 13(6):612–32. doi: 10.1177/1747493018778713 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

