Impact of a multi-strain probiotic administration on peri-rectal colonization with drug-resistant Gram-negative bacteria in preterm neonates
- PMID: 36405834
- PMCID: PMC9667553
- DOI: 10.3389/fped.2022.1002762
Impact of a multi-strain probiotic administration on peri-rectal colonization with drug-resistant Gram-negative bacteria in preterm neonates
Abstract
Background: Infections caused by drug resistant Gram-negative bacteria (DR-GNB) are a major health concern for hospitalized preterm neonates, globally. The aim of this study was to investigate the effect of a multi-strain probiotic on the incidence of rectal colonization with DR-GNB in preterm neonates.
Methods: A double-blind, placebo-controlled, randomized clinical trial was conducted including 200 neonates, randomly allocated to a multi-strain probiotic (n = 100) or placebo (n = 100).
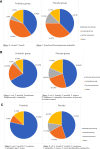
Results: Fifteen percent of the neonates showed peri-rectal colonization with DR-GNB on the day of enrolment indicating probable maternal-to-neonate (vertical) bacterial transmission or environmental acquisition at time of delivery, with no difference between groups. Acquisition of further DR-GNB colonization was rapid, with an increase from 15% on the day enrolment to 77% by day 7 and 83% by day 14 of life. By day 7 (corresponding to early gut colonization), neonates in the probiotic group were 57% less likely to have peri-rectal DR-GNB colonization [OR: 0.43 (0.20-0.95); p = 0.04] and by day 14 (corresponding to late gut colonization), neonates in the probiotic group were 93% less likely to have peri-rectal DR-GNB colonization [OR: 0.07 (0.02-0.23); p < 0.001].
Conclusion: Hospitalized neonates showed substantial peri-rectal colonization with DR-GNB at enrolment and further rapid acquisition of DR-GNB in the first 2 weeks of life. The use of a multi-strain probiotic was effective in reducing early and late neonatal gut colonization with DR-GNB.
Clinical trial registration: The trial was registered at the Pan African Clinical Trial Registry (PACTR202011513390736).
Keywords: multidrug-resistant Gram negative bacilli; neonate; premature (babies); probiotic; rectal swab.
© 2022 Sowden, van Niekerk, Bulabula, Dramowski, Whitelaw, Twisk and Van Weissenbruch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Harder T, Seidel J, Eckmanns T, Weiss B, Haller S. Predicting late-onset sepsis by routine neonatal screening for colonisation by Gram-negative bacteria in neonates at intensive care units: a protocol for a systematic review. BMJ Open. (2017) 7(3):1–7. 10.1136/bmjopen-2016-014986 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

