The ABBA project (Assess Better Before Access): A retrospective cohort study of neonatal intravascular device outcomes
- PMID: 36405839
- PMCID: PMC9670536
- DOI: 10.3389/fped.2022.980725
The ABBA project (Assess Better Before Access): A retrospective cohort study of neonatal intravascular device outcomes
Abstract
Background: Venous access devices (VADs) play a vital role within the neonatal intensive care unit. However, there are significant risks associated with the use of VADs, with complications such as infection, thrombosis, device occlusion, and infiltration/extravasation frequently contributing to device-related failures and increasing the risk of significant patient harm or injury. This study aimed to explore the relationships between risk factors and different venous access device complications in the neonatal setting, and then use that evidence to develop an algorithm based on observational data.
Methods: This is a retrospective, single-center cohort study that was conducted in a large 112-bed neonatal intensive care unit in Qatar. We examined venous access device data from January 2016 to December 2018 for all term and preterm neonates. Descriptive statistics were used to summarize the outcomes, which included a mean and its standard deviation or median and an interquartile range for continuous variables regarding normal distribution, and absolute numbers with percentages for discrete variables.
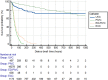
Results: The authors recorded a total of 23,858 VADs inserted during the study period. Of these, 21,313 (89%) were peripheral intravenous catheters, 689 (3%) were extended dwell-peripheral intravenous catheters, 1,335 (6%) were epicutaneo-caval catheters, and 521 (2%) were umbilical venous catheters. In total, 51,179 catheter days were registered, with 2.17 catheter days reported per patient. Peripheral device dwell times were significantly shorter when compared with central venous catheter devices (P < 0.001), with mean dwell times of 22 days ± 23 h and 236 days ± 183 h, respectively. After insertion, a complication occurred in 11,177 (51%) of peripheral VADs and 221 (12%) of central VADs. The type of device inserted [P < 0.001, hazard ratio (HR) = 0.52, 95% confidence interval (CI): 0.50-0.54], reason/indication for intravenous therapy (P < 0.001, HR = 0.85, 95% CI: 0.82-0.87), and the side of insertion of the device (P < 0.001, HR = 1.25, 95% CI: 1.24-1.27) had a significant relationship with outcomes.
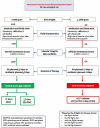
Conclusions: Four subgroups of VADs were identified (peripheral intravenous catheters, extended dwell-peripheral intravenous devices, epicutaneo-caval catheters, and umbilical venous catheters) with outcome-related differences. Central venous access devices (epicutaneo-caval catheters and umbilical venous catheters) had lower complications compared with peripheral VADs. Proper venous access device selection, early insertion, and early removal approaches remain crucial to preventing venous access device complications. Peripheral intravenous devices should be used carefully and closely watched for early detection of complications.
Keywords: NICU; central venous catheters; device-related complications; epicutaneo-caval catheter; intravenous therapy; neonate; peripheral intravenous devices; peripherally inserted central catheter.
© 2022 Van Rens, Bayoumi, van den Hoogen, Francia, Cabanillas, Van Loon and Spencer.
Conflict of interest statement
TRS was employed by company Global Vascular Access, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Extended Dwell Peripheral Intravenous Catheter Is an Alternative Method of NICU Intravenous Access.Adv Neonatal Care. 2018 Aug;18(4):295-301. doi: 10.1097/ANC.0000000000000515. Adv Neonatal Care. 2018. PMID: 29847401 Free PMC article.
-
Effect of implementing an Epicutaneo-Caval Catheter team in Neonatal Intensive Care Unit.J Vasc Access. 2021 Mar;22(2):243-253. doi: 10.1177/1129729820928182. Epub 2020 Jun 30. J Vasc Access. 2021. PMID: 32602399 Free PMC article.
-
Efficacy of antimicrobial-impregnated catheters in preventing sepsis post epicutaneo-caval catheter (ECC) removal in neonates: A retrospective study.J Vasc Access. 2024 Sep 26:11297298241281640. doi: 10.1177/11297298241281640. Online ahead of print. J Vasc Access. 2024. PMID: 39327717
-
Use of tissue adhesive for neonatal intravenous access devices: A scoping review.Eur J Pediatr. 2024 Dec;183(12):5103-5112. doi: 10.1007/s00431-024-05800-3. Epub 2024 Oct 5. Eur J Pediatr. 2024. PMID: 39367917 Free PMC article.
-
Midline Catheter Use in the Neonatal Intensive Care Unit.Crit Care Nurs Clin North Am. 2024 Mar;36(1):111-118. doi: 10.1016/j.cnc.2023.09.004. Epub 2023 Oct 23. Crit Care Nurs Clin North Am. 2024. PMID: 38296369 Review.
Cited by
-
The Modern Role of Neonatal PICCs Subspecialty.Nurs Crit Care. 2025 Jul;30(4):e70111. doi: 10.1111/nicc.70111. Nurs Crit Care. 2025. PMID: 40611458 Free PMC article. Review.
-
Octyl-butyl-cyanoacrylate glue for securement of peripheral intravenous catheters: A retrospective, observational study in the neonatal population.J Vasc Access. 2024 Jul;25(4):1229-1237. doi: 10.1177/11297298231154629. Epub 2023 Feb 16. J Vasc Access. 2024. PMID: 36794683 Free PMC article.
-
Peripheral intravenous therapy infiltration and extravasation (PIVIE) risks in 11 006 paediatric surgery inpatients in China: a retrospective observational study.J Int Med Res. 2024 Oct;52(10):3000605241283600. doi: 10.1177/03000605241283600. J Int Med Res. 2024. PMID: 39382036 Free PMC article.
-
Prevention of complications related to peripherally inserted central catheter insertion techniques in newborns: systematic review and network meta-analysis.Rev Lat Am Enfermagem. 2024 Jul 5;32:e4161. doi: 10.1590/1518-8345.6905.4161. eCollection 2024. Rev Lat Am Enfermagem. 2024. PMID: 38985042 Free PMC article.
-
Short versus long peripheral intravenous catheters in neonates: a retrospective cohort study.Sci Rep. 2025 May 2;15(1):15373. doi: 10.1038/s41598-025-00301-1. Sci Rep. 2025. PMID: 40316560 Free PMC article.
References
-
- van Rens MFPT, Hugill K, Mahmah MA, van Rens MF, Hugill K, Mahmah MA, et al. Evaluation of unmodifiable and potentially modifiable factors affecting peripheral intravenous device-related complications in neonates: a retrospective observational study. BMJ Open. (2021) 11(9):e047788. 10.1136/bmjopen-2020-047788 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

