Current landscape of clinical trials for HPV-positive head and neck squamous cell carcinoma (HNSCC)
- PMID: 36405940
- PMCID: PMC9666285
- DOI: 10.3332/ecancer.2022.1447
Current landscape of clinical trials for HPV-positive head and neck squamous cell carcinoma (HNSCC)
Abstract
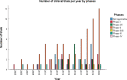
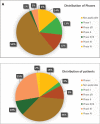
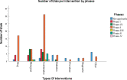
This study aims to determine the current state of clinical trials regarding HPV-positive head and neck squamous cell carcinoma (HNSCC). Clinical trials were filtered to fit the study's aim using Clinicaltrials.gov: trials concerning HNSCC specifically those related to HPV done between January 2005 and December 2020 were extracted and information regarding location, duration, phases, patient recruitment, trial status, results, primary outcome, type of intervention and publication status were collected and analysed. As a result, 123 trials were included. North American countries (USA and Canada) conducted more than two-thirds of the trials (72.4%) compared to European countries and the rest of the world. Trials in phase II constituted more than half of those included in this study (53.7%). From the 123 trials included in this study, only 30 had their NCT identification number linked to publications, but less than half (46.7%) of the publications stemmed from trials with results. Drug combination was the most widely studied treatment modality. Despite falling in the middle of the spectrum with respect to the number of trials when compared to other diseases, our research highlights the need for even more trials tackling multiple aspects of HPV-positive HNSCC.
Keywords: HPV-positive; chemoradiotherapy; chemotherapy; clinical trials; head and neck squamous cell cancer.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis.Front Immunol. 2021 Apr 7;12:645170. doi: 10.3389/fimmu.2021.645170. eCollection 2021. Front Immunol. 2021. PMID: 33897693 Free PMC article.
-
TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma.Cancer. 2017 May 15;123(10):1778-1790. doi: 10.1002/cncr.30570. Epub 2017 Mar 13. Cancer. 2017. PMID: 28295222 Free PMC article.
-
Disease outcome and associated factors after definitive platinum based chemoradiotherapy for advanced stage HPV-negative head and neck cancer.Radiother Oncol. 2022 Oct;175:112-121. doi: 10.1016/j.radonc.2022.08.013. Epub 2022 Aug 13. Radiother Oncol. 2022. PMID: 35973619
-
Clinico-pathological peculiarities of human papilloma virus driven head and neck squamous cell carcinoma: A comprehensive update.Life Sci. 2020 Mar 15;245:117383. doi: 10.1016/j.lfs.2020.117383. Epub 2020 Jan 30. Life Sci. 2020. PMID: 32007572 Review.
-
EGFR overexpression increases radiotherapy response in HPV-positive head and neck cancer through inhibition of DNA damage repair and HPV E6 downregulation.Cancer Lett. 2021 Feb 1;498:80-97. doi: 10.1016/j.canlet.2020.10.035. Epub 2020 Oct 31. Cancer Lett. 2021. PMID: 33137407
Cited by
-
Interventions for the treatment of oral cavity and oropharyngeal cancers: surgical treatment.Cochrane Database Syst Rev. 2023 Aug 31;8(8):CD006205. doi: 10.1002/14651858.CD006205.pub5. Cochrane Database Syst Rev. 2023. PMID: 37650478 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials