Intranasal delivery of a chimpanzee adenovirus vector expressing a pre-fusion spike (BV-AdCoV-1) protects golden Syrian hamsters against SARS-CoV-2 infection
- PMID: 36405962
- PMCID: PMC9671113
- DOI: 10.3389/fcimb.2022.979641
Intranasal delivery of a chimpanzee adenovirus vector expressing a pre-fusion spike (BV-AdCoV-1) protects golden Syrian hamsters against SARS-CoV-2 infection
Abstract
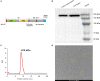
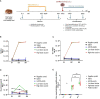
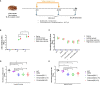
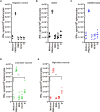
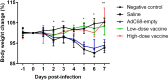
We evaluated the immunogenicity and protective ability of a chimpanzee replication-deficient adenovirus vectored COVID-19 vaccine (BV-AdCoV-1) expressing a stabilized pre-fusion SARS-CoV-2 spike glycoprotein in golden Syrian hamsters. Intranasal administration of BV-AdCoV-1 elicited strong humoral and cellular immunity in the animals. Furthermore, vaccination prevented weight loss, reduced SARS-CoV-2 infectious virus titers in the lungs as well as lung pathology and provided protection against SARS-CoV-2 live challenge. In addition, there was no vaccine-induced enhanced disease nor immunopathological exacerbation in BV-AdCoV-1-vaccinated animals. Furthermore, the vaccine induced cross-neutralizing antibody responses against the ancestral strain and the B.1.617.2, Omicron(BA.1), Omicron(BA.2.75) and Omicron(BA.4/5) variants of concern. These results demonstrate that BV-AdCoV-1 is potentially a promising candidate vaccine to prevent SARS-CoV-2 infection, and to curtail pandemic spread in humans.
Keywords: COVID-19 vaccine; Chimpanzee Adenovirus Serotype 68; challenge study; golden Syrian hamsters; intranasal.
Copyright © 2022 Wang, Xu, Mu, Qin, Zhao, Xie, Du, Wu, Legrand, Mouchain, Fichet, Liu, Yin, Zhao, Ji, Gong, Klein and Wu.
Conflict of interest statement
Author SW, LXu, TM, MQ, PZ, LXi, YW, YL, WY, JZ, MJ, BG, MK, and KW are employed by Wuhan BravoVax Co., Ltd. MK and KW are also employed by Shanghai BravoVax Co., Ltd. Meanwhile, YL is a teacher from Hubei University. LD is employed by Voisin Consulting Life Sciences. NL, KM, and GF are employed by Oncodesign. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Aerosol Inhalation of Chimpanzee Adenovirus Vectors (ChAd68) Expressing Ancestral or Omicron BA.1 Stabilized Pre-Fusion Spike Glycoproteins Protects Non-Human Primates against SARS-CoV-2 Infection.Vaccines (Basel). 2023 Aug 28;11(9):1427. doi: 10.3390/vaccines11091427. Vaccines (Basel). 2023. PMID: 37766104 Free PMC article.
-
Efficacy of parainfluenza virus 5 (PIV5)-vectored intranasal COVID-19 vaccine as a single dose primer and booster against SARS-CoV-2 variants.J Virol. 2025 Apr 15;99(4):e0198924. doi: 10.1128/jvi.01989-24. Epub 2025 Mar 21. J Virol. 2025. PMID: 40116505 Free PMC article.
-
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge.PLoS Pathog. 2025 Apr 21;21(4):e1012585. doi: 10.1371/journal.ppat.1012585. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40258004 Free PMC article.
-
[Research and application of the SARS-CoV-2 vaccine based on adenovirus vector technology platform].Zhonghua Yu Fang Yi Xue Za Zhi. 2023 Jul 6;57(7):1082-1095. doi: 10.3760/cma.j.cn112150-20230419-00309. Zhonghua Yu Fang Yi Xue Za Zhi. 2023. PMID: 37198717 Review. Chinese.
-
The Adenovirus Vector Platform: Novel Insights into Rational Vector Design and Lessons Learned from the COVID-19 Vaccine.Viruses. 2023 Jan 11;15(1):204. doi: 10.3390/v15010204. Viruses. 2023. PMID: 36680244 Free PMC article. Review.
Cited by
-
An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission.Nat Commun. 2024 Feb 2;15(1):995. doi: 10.1038/s41467-024-45348-2. Nat Commun. 2024. PMID: 38307868 Free PMC article.
-
Intranasal vaccination with an NDV-vectored SARS-CoV-2 vaccine protects against Delta and Omicron challenges.NPJ Vaccines. 2024 May 23;9(1):90. doi: 10.1038/s41541-024-00870-8. NPJ Vaccines. 2024. PMID: 38782986 Free PMC article.
-
Application of spatial transcriptomics analysis using the Visium system for the mouse nasal cavity after intranasal vaccination.Front Immunol. 2023 Jul 21;14:1209945. doi: 10.3389/fimmu.2023.1209945. eCollection 2023. Front Immunol. 2023. PMID: 37545501 Free PMC article.
-
Aerosol Inhalation of Chimpanzee Adenovirus Vectors (ChAd68) Expressing Ancestral or Omicron BA.1 Stabilized Pre-Fusion Spike Glycoproteins Protects Non-Human Primates against SARS-CoV-2 Infection.Vaccines (Basel). 2023 Aug 28;11(9):1427. doi: 10.3390/vaccines11091427. Vaccines (Basel). 2023. PMID: 37766104 Free PMC article.
References
-
- Bednash J. S., Kagan V. E., Englert J. A., Farkas D., Tyurina Y. Y., Tyurin V. A., et al. . (2022). Syrian Hamsters as a model of lung injury with SARS-CoV-2 infection: Pathologic, physiologic, and detailed molecular profiling. Transl. Res. 240, 1–16. doi: 10.1016/j.trsl.2021.10.007 - DOI - PMC - PubMed
-
- Bennett J. V., Castro J. F. D., Valdespino-Gomez J. L., Garcia-Garcia M. D. L., Islas-Romero R., Echaniz-Aviles G., et al. . (2002). Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: Randomized trials in Mexican school children. Bull. W. H. O. 80 (10), 806–812. doi: 10.1590/S0042-96862002001000009 - DOI - PMC - PubMed
-
- Boudewijns R., Pérez P., Lázaro-Frías A., Looveren D. V., Vercruysse T., Thibaut H. J., et al. . (2022). MVA-CoV2-S vaccine candidate neutralizes distinct variants of concern and protects against SARS-CoV-2 infection in hamsters. Front. Immunol. 13. doi: 10.3389/fimmu.2022.845969 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

