The lipidome of Crithidia fasiculata and its plasticity
- PMID: 36405970
- PMCID: PMC9671073
- DOI: 10.3389/fcimb.2022.945750
The lipidome of Crithidia fasiculata and its plasticity
Abstract
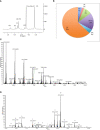
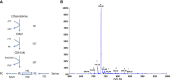
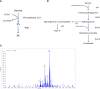
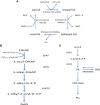
Crithidia fasiculata belongs to the trypanosomatidae order of protozoan parasites, bearing close relation to other kinetoplastid parasites such as Trypanosoma brucei and Leishmania spp. As an early diverging lineage of eukaryotes, the study of kinetoplastid parasites has provided unique insights into alternative mechanisms to traditional eukaryotic metabolic pathways. Crithidia are a monogenetic parasite for mosquito species and have two distinct lifecycle stages both taking place in the mosquito gut. These consist of a motile choanomastigote form and an immotile amastigote form morphologically similar to amastigotes in Leishmania. Owing to their close relation to Leishmania, Crithidia are a growing research tool, with continuing interest in its use as a model organism for kinetoplastid research with the added benefit that they are non-pathogenic to humans and can be grown with no special equipment or requirements for biological containment. Although comparatively little research has taken place on Crithidia, similarities to other kinetoplast species has been shown in terms of energy metabolism and genetics. Crithidia also show similarities to kinetoplastids in their production of the monosaccharide D-arabinopyranose similar to Leishmania, which is incorporated into a lipoarabinogalactan a major cell surface GPI-anchored molecule. Additionally, Crithidia have been used as a eukaryotic expression system to express proteins from other kinetoplastids and potentially other eukaryotes including human proteins allowing various co- and post-translational protein modifications to the recombinant proteins. Despite the obvious usefulness and potential of this organism very little is known about its lipid metabolism. Here we describe a detailed lipidomic analyses and demonstrate the possible placidity of Crithidia's lipid metabolis. This could have important implications for biotechnology approaches and how other kinetoplastids interact with, and scavenge nutrients from their hosts.
Keywords: Crithidia; fatty acids; kinetoplastid; lipids; oils; plasticity; sugars.
Copyright © 2022 Cerone, Roberts and Smith.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Distinct genes encode type II Topoisomerases for the nucleus and mitochondrion in the protozoan parasite Trypanosoma brucei.J Biol Chem. 2006 Feb 10;281(6):3048-56. doi: 10.1074/jbc.M505977200. Epub 2005 Nov 28. J Biol Chem. 2006. PMID: 16316982
-
The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets.Molecules. 2020 Nov 5;25(21):5155. doi: 10.3390/molecules25215155. Molecules. 2020. PMID: 33167520 Free PMC article. Review.
-
2,3-Diphenyl-2,3-dihydro-4H-1,3-thiaza-4-one heterocycles inhibit growth and block completion of cytokinesis in kinetoplastid parasites.Mol Biochem Parasitol. 2021 Sep;245:111396. doi: 10.1016/j.molbiopara.2021.111396. Epub 2021 Jul 21. Mol Biochem Parasitol. 2021. PMID: 34302898
-
The biology of kinetoplastid parasites: insights and challenges from genomics and post-genomics.Int J Parasitol. 2001 May 1;31(5-6):443-52. doi: 10.1016/s0020-7519(01)00154-0. Int J Parasitol. 2001. PMID: 11334928 Review.
-
Glycosyl-phosphatidylinositol molecules of the parasite and the host.Parasitology. 1994;108 Suppl:S45-54. doi: 10.1017/s0031182000075715. Parasitology. 1994. PMID: 8084654 Review.
Cited by
-
Building a cell-factory in Crithidia fasciculata: a bio-sustainable system to produce high-value polyunsaturated fatty acids.Microb Cell Fact. 2025 Jun 23;24(1):142. doi: 10.1186/s12934-025-02760-7. Microb Cell Fact. 2025. PMID: 40551153 Free PMC article.
-
Exploring the activity of the putative Δ6-desaturase and its role in bloodstream form life-cycle transitions in Trypanosoma brucei.PLoS Pathog. 2025 Feb 18;21(2):e1012691. doi: 10.1371/journal.ppat.1012691. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39965027 Free PMC article.
References
-
- Acosta-Serrano A., Vassella E., Liniger M., Renggli Kunz C., Brun R., Roditi I., et al. . (2001). The surface coat of procyclic trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proc. Natl. Acad. Sci. U.S.A. 98, 1513–1518. doi: 10.1073/pnas.98.4.1513 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

