Promoting oral mucosal wound healing using a DCS-RuB2A2 hydrogel based on a photoreactive antibacterial and sustained release of BMSCs
- PMID: 36406253
- PMCID: PMC9650008
- DOI: 10.1016/j.bioactmat.2022.10.027
Promoting oral mucosal wound healing using a DCS-RuB2A2 hydrogel based on a photoreactive antibacterial and sustained release of BMSCs
Abstract
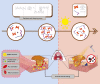
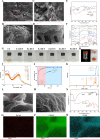
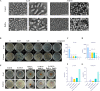
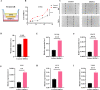
The high occurrence rate and difficulties in symptom control are listed as the major problems of oral mucosal disease by medical professionals. Following the development of oral mucosal lesions, the oral microenvironment changes, immunity declines, and continuous bacterial stimulation causes wound infection. Traditional antibacterial drugs are ineffective for oral mucosal lesions. To overcome this problem, a light-responsive antibacterial hydrogel containing sustained-release BMSCs was inspired by the trauma environment in the oral cavity, which is different from that on the body surface since it mostly remains under dark conditions. In the absence of light, the hydrogel seals the wound to form a barrier, exerts a natural bacteriostatic effect, and prevents invasion by foreign bacteria. Simultaneously, mesenchymal stem cells are presented, and the released growth factors and other substances have excellent anti-inflammatory and angiogenic effects, which result in rapid repair of the damaged site. Under light conditions, after photo-induced shedding of the hydrogel, RuB2A exerts an antibacterial effect accompanied by degradation of the hydrogel. Results in a rat oral mucosal repair model demonstrate that DCS-RuB2A2-BMSCs could rapidly repair the oral mucosa within 4 days. Sequencing data provide ideas for further analysis of the intrinsic molecular mechanisms and signaling pathways. Taken together, our results suggest that this light-responsive antibacterial hydrogel loaded with BMSCs can be used for rapid wound repair and may advance the development of therapeutic strategies for the treatment of clinical oral mucosal defects.
Keywords: BMSCs; DCS; Hydrogel; Light-responsive; Oral mucosa.
© 2022 The Authors.
Figures




Similar articles
-
In situ photo-crosslinked hydrogel promotes oral mucosal wound healing through sustained delivery of ginsenoside Rg1.Front Bioeng Biotechnol. 2023 Sep 28;11:1252574. doi: 10.3389/fbioe.2023.1252574. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37840668 Free PMC article.
-
Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa.Acta Biomater. 2019 Dec;100:255-269. doi: 10.1016/j.actbio.2019.10.011. Epub 2019 Oct 10. Acta Biomater. 2019. PMID: 31606531
-
Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury.Acta Biomater. 2021 Sep 1;131:185-197. doi: 10.1016/j.actbio.2021.06.038. Epub 2021 Jul 1. Acta Biomater. 2021. PMID: 34217903
-
Development of a visible light, cross-linked GelMA hydrogel containing decellularized human amniotic particles as a soft tissue replacement for oral mucosa repair.RSC Adv. 2019 Jun 11;9(32):18344-18352. doi: 10.1039/c9ra03009c. eCollection 2019 Jun 10. RSC Adv. 2019. PMID: 35547651 Free PMC article.
-
NIR-activated multi-hit therapeutic Ag2S quantum dot-based hydrogel for healing of bacteria-infected wounds.Acta Biomater. 2022 Jun;145:88-105. doi: 10.1016/j.actbio.2022.04.013. Epub 2022 Apr 14. Acta Biomater. 2022. PMID: 35429669
Cited by
-
All-in-One: A Multifunctional Composite Biomimetic Cryogel for Coagulation Disorder Hemostasis and Infected Diabetic Wound Healing.Nanomicro Lett. 2025 Mar 3;17(1):171. doi: 10.1007/s40820-024-01603-1. Nanomicro Lett. 2025. PMID: 40025402 Free PMC article.
-
Histopathological Evaluation of Wound Healing and Anti-Inflammatory Effects of Granola Potato Peel Ethanol Extract in Rat Oral Mucosa.J Exp Pharmacol. 2024 Oct 24;16:377-395. doi: 10.2147/JEP.S487373. eCollection 2024. J Exp Pharmacol. 2024. PMID: 39469135 Free PMC article.
-
Wet adhesive hydrogels based on niobium carbide for experimental research of oral mucosal impairment.RSC Adv. 2024 Apr 22;14(18):12935-12946. doi: 10.1039/d4ra01352b. eCollection 2024 Apr 16. RSC Adv. 2024. PMID: 38650683 Free PMC article.
-
A cell-free SHED lysate-hydrogel system for oral ulcer healing with anti-inflammatory and pro-angiogenic effects.J Nanobiotechnology. 2025 Jul 28;23(1):547. doi: 10.1186/s12951-025-03597-3. J Nanobiotechnology. 2025. PMID: 40722154 Free PMC article.
-
In situ photo-crosslinked hydrogel promotes oral mucosal wound healing through sustained delivery of ginsenoside Rg1.Front Bioeng Biotechnol. 2023 Sep 28;11:1252574. doi: 10.3389/fbioe.2023.1252574. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37840668 Free PMC article.
References
LinkOut - more resources
Full Text Sources

