Bactericidal Efficacy of Nanostructured Surfaces Increases under Flow Conditions
- PMID: 36406483
- PMCID: PMC9670296
- DOI: 10.1021/acsomega.2c05828
Bactericidal Efficacy of Nanostructured Surfaces Increases under Flow Conditions
Abstract
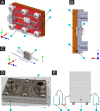
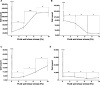
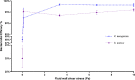
Bacterial colonization on solid surfaces creates enormous problems across various industries causing billions of dollars' worth of economic damages and costing human lives. Biomimicking nanostructured surfaces have demonstrated a promising future in mitigating bacterial colonization and related issues. The importance of this non-chemical method has been elevated due to bacterial evolvement into antibiotic and antiseptic-resistant strains. However, bacterial attachment and viability on nanostructured surfaces under fluid flow conditions has not been investigated thoroughly. In this study, attachment and viability of Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus) on a model nanostructured surface were studied under fluid flow conditions. A wide range of flow rates resulting in a broad spectrum of fluid wall shear stress on a nanostructured surface representing various application conditions were experimentally investigated. The bacterial suspension was pumped through a custom-designed microfluidic device (MFD) that contains a sterile Ti-6Al-4V substrate. The surface of the titanium substrate was modified using a hydrothermal synthesis process to fabricate the nanowire structure on the surface. The results of the current study show that the fluid flow significantly reduces bacterial adhesion onto nanostructured surfaces and significantly reduces the viability of adherent cells. Interestingly, the bactericidal efficacy of the nanostructured surface was increased under the flow by ∼1.5-fold against P. aeruginosa and ∼3-fold against S. aureus under static conditions. The bactericidal efficacy had no dependency on the fluid wall shear stress level. However, trends in the dead-cell count with the fluid wall shear were slightly different between the two species. These findings will be highly useful in developing and optimizing nanostructures in the laboratory as well as translating them into successful industrial applications. These findings may be used to develop antibacterial surfaces on biomedical equipment such as catheters and vascular stents or industrial applications such as ship hulls and pipelines where bacterial colonization is a great challenge.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Brown L. M.; McComb J. P.; Vangsness M. D.; Bowen L. L.; Mueller S. S.; Balster L. M.; Bleckmann C. A. Community Dynamics and Phylogenetics of Bacteria Fouling Jet A and JP-8 Aviation Fuel. Int. Biodeterior. Biodegrad. 2010, 64, 253–261. 10.1016/j.ibiod.2010.01.008. - DOI
LinkOut - more resources
Full Text Sources

