Robust Antiviral Activity of Santonica Flower Extract (Artemisia cina) against Avian and Human Influenza A Viruses: In Vitro and Chemoinformatic Studies
- PMID: 36406485
- PMCID: PMC9670689
- DOI: 10.1021/acsomega.2c04867
Robust Antiviral Activity of Santonica Flower Extract (Artemisia cina) against Avian and Human Influenza A Viruses: In Vitro and Chemoinformatic Studies
Abstract
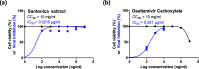
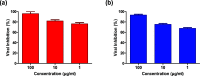
The evolution of drug-resistant viral strains following natural acquisition of resistance mutations is a major obstacle to antiviral therapy. Besides the improper prescription of the currently licensed anti-influenza medications, M2-blockers and neuraminidase inhibitors, to control poultry outbreaks/infections potentiates the emergence of drug-resistant influenza variants. Therefore, there is always a necessity to find out new alternatives with potent activity and high safety. Plant extracts and plant-based chemicals represent a historical antiviral resource with remarkable safety in vitro and in vivo to control the emerging and remerging health threats caused by viral infections. Herein, a panel of purified plant extracts and subsequent plant-derived chemicals were evaluated for their anti-avian influenza activity against zoonotic highly pathogenic influenza A/H5N1 virus. Interestingly, santonica flower extract (Artemisia cina) showed the most promising anti-H5N1 activity with a highly safe half-maximal cytotoxic concentration 50 (CC50 > 10 mg/mL) and inhibitory concentration 50 (IC50 of 3.42 μg/mL). To confirm the anti-influenza activity, we assessed the anti-influenza activity of the selected plant extracts against seasonal human influenza A/H1N1 virus and we found that santonica flower extract showed a robust anti-influenza activity that was comparable to the activity against influenza A/H5N1. Furthermore, the mode of action for santonica flower extract with strong inhibitory activity on the abovementioned influenza strains was elucidated, showing a virucidal effect. To go deeper about the activity of the chemometric component of the extract, the major constituent, santonin, was further selected for in vitro screening against influenza A/H5N1 (IC50 = 1.701 μg/mL) and influenza A/H1N1 (IC50 = 2.91 μg/mL). The oxygen of carbonyl functionality in the cyclohexene ring succeeded to form a hydrogen bond with the neuraminidase active site. Despite the fact that santonin revealed similarity to both reference neuraminidase inhibitors in forming hydrogen bonds with essential amino acids, it illustrated shape alignment to oseltamivir more than zanamivir according to Tanimoto algorithms. This study highlights the applicability of santonica flower extract as a promising natural antiviral against low and highly pathogenic influenza A viruses.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Richard M.; van den Brand J. M. A.; Bestebroer T. M.; Lexmond P.; de Meulder D.; Fouchier R. A. M.; Lowen A. C.; Herfst S. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat. Commun. 2020, 11, 766. 10.1038/s41467-020-14626-0. - DOI - PMC - PubMed
- Mostafa A.; Abdelwhab E. M.; Mettenleiter T. C.; Pleschka S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. 10.3390/v10090497. - DOI - PMC - PubMed
-
- Spackman E., A Brief Introduction to Avian Influenza Virus. In Animal Influenza Virus: Methods and Protocols, Spackman E., Ed. Springer US: New York, NY, 2020; pp. 83–92. - PubMed
-
- Tenforde M. W.; Kondor R. J. G.; Chung J. R.; Zimmerman R. K.; Nowalk M. P.; Jackson M. L.; Jackson L. A.; Monto A. S.; Martin E. T.; Belongia E. A.; McLean H. Q.; Gaglani M.; Rao A.; Kim S. S.; Stark T. J.; Barnes J. R.; Wentworth D. E.; Patel M. M.; Flannery B. Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019-2020. Clin Infect Dis 2021, 73, e4244–e4250. 10.1093/cid/ciaa1884. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

