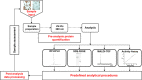
Bioanalytical Workflow for Qualitative and Quantitative Assessment of Hot-Melt Extruded Lysozyme Formulations
- PMID: 36406547
- PMCID: PMC9670096
- DOI: 10.1021/acsomega.2c03559
Bioanalytical Workflow for Qualitative and Quantitative Assessment of Hot-Melt Extruded Lysozyme Formulations
Abstract
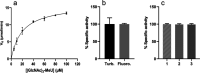
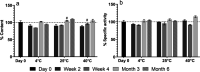
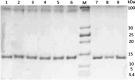
Structural and functional integrities of formulated proteins are key characteristics that provide a better understanding of influencing factors and their adjustment during formulation development. Here, the procedures commonly used for protein analysis were applied and optimized to obtain a higher degree of accuracy, reproducibility, and reliability for the analysis of lysozyme extracts from hot-melt extrudates (HME). The extrudates were prepared with polyethylene glycol 20 000. The test lysozyme HMEs were subjected to extraction procedures and analytical methods following the International Council of Harmonization guidelines for testing the active protein ingredient Q 1 A (R2) in its pure and formulated form. Therefore, reversed-phase high-pressure liquid chromatography, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, matrix-assisted laser desorption ionization mass spectrometry, and fluorescence-based activity measurements were applied to study lysozyme stability and function after formulation. Long-term accelerated stability studies were performed for the pure and formulated protein. Our findings revealed a high degree of stability for lysozyme toward different temperatures and storage times, confirming that HME is a suitable formulation alternative that preserves lysozyme's properties and stability. The presented methods and workflow are recommended to be exploited for further protein drugs to assess usability and compatibility concerning different pharmaceutical applications.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

