Neutrophil extracellular traps contribute to myofibroblast differentiation and scar hyperplasia through the Toll-like receptor 9/nuclear factor Kappa-B/interleukin-6 pathway
- PMID: 36406661
- PMCID: PMC9668674
- DOI: 10.1093/burnst/tkac044
Neutrophil extracellular traps contribute to myofibroblast differentiation and scar hyperplasia through the Toll-like receptor 9/nuclear factor Kappa-B/interleukin-6 pathway
Abstract
Background: Inflammation is an important factor in pathological scarring. The role of neutrophils, one of the most important inflammatory cells, in scar hyperplasia remains unclear. The purpose of this article is to study the correlation between neutrophil extracellular traps (NETs) and scar hyperplasia and identify a new target for inhibiting scar hyperplasia.
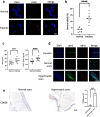
Methods: Neutrophils were isolated from human peripheral blood by magnetic-bead sorting. NETs in plasma and scars were detected by enzyme-linked immunosorbent assays (ELISAs), immunofluorescence and flow cytometry. Immunohistochemistry was used to assess neutrophil (CD66B) infiltration in hypertrophic scars. To observe the entry of NETs into fibroblasts we used immunofluorescence and flow cytometry.
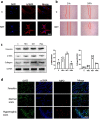
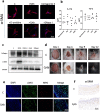
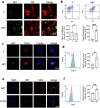
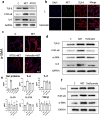
Results: We found that peripheral blood neutrophils in patients with hypertrophic scars were more likely to form NETs (p < 0.05). Hypertrophic scars showed greater infiltration with neutrophils and NETs (p < 0.05). NETs activate fibroblasts in vitro to promote their differentiation and migration. Inhibition of NETs with cytochalasin in wounds reduced the hyperplasia of scars in mice. We induced neutrophils to generate NETs with different stimuli in vitro and detected the proteins carried by NETs. We did not find an increase in the expression of common scarring factors [interleukin (IL)-17 and transforming growth factor-β (TGF-β), p > 0.05]. However, inhibiting the production of NETs or degrading DNA reduced the differentiation of fibroblasts into myofibroblasts. In vitro, NETs were found to be mediated by Toll-like receptor 9 (TLR-9) in fibroblasts and further phosphorylated nuclear factor Kappa-B (NF-κB). We found that IL-6, which is downstream of NF-κB, was increased in fibroblasts. Additionally, IL-6 uses autocrine and paracrine signaling to promote differentiation and secretion.
Conclusions: Our experiments found that NETs activate fibroblasts through the TLR-9/NF-κB/IL-6 pathway, thereby providing a new target for regulating hypertrophic scars.
Keywords: Fibroblast, Inflammation, Differentiation, Nuclear factor Kappa-B, Interleukin-6; Hypertrophic scar; Neutrophil extracellular traps; Toll-like receptor 9.
© The Author(s) 2022. Published by Oxford University Press.
Figures
Similar articles
-
Neutrophil extracellular traps promote differentiation and function of fibroblasts.J Pathol. 2014 Jul;233(3):294-307. doi: 10.1002/path.4359. Epub 2014 May 19. J Pathol. 2014. PMID: 24740698
-
PSTPIP2 ameliorates aristolochic acid nephropathy by suppressing interleukin-19-mediated neutrophil extracellular trap formation.Elife. 2024 Feb 5;13:e89740. doi: 10.7554/eLife.89740. Elife. 2024. PMID: 38314821 Free PMC article.
-
Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages.Cell Cycle. 2019 Nov;18(21):2928-2938. doi: 10.1080/15384101.2019.1662678. Epub 2019 Sep 8. Cell Cycle. 2019. PMID: 31496351 Free PMC article.
-
Control of wound contraction. Basic and clinical features.Hand Clin. 2000 May;16(2):289-302. Hand Clin. 2000. PMID: 10791174 Review.
-
Acne-induced pathological scars: pathophysiology and current treatments.Burns Trauma. 2024 Apr 5;12:tkad060. doi: 10.1093/burnst/tkad060. eCollection 2024. Burns Trauma. 2024. PMID: 38585341 Free PMC article. Review.
Cited by
-
Pentatrichomonas hominis induces extracellular traps formation of macrophages via the TLR2/NADPH/PAD4 pathway.Parasit Vectors. 2025 Jul 1;18(1):248. doi: 10.1186/s13071-025-06840-w. Parasit Vectors. 2025. PMID: 40598534 Free PMC article.
-
CLC-3 regulates TGF-β/smad signaling pathway to inhibit the process of fibrosis in hypertrophic scar.Heliyon. 2024 Jan 19;10(3):e24984. doi: 10.1016/j.heliyon.2024.e24984. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333829 Free PMC article.
-
Neutrophil extracellular traps promoting fibroblast activation and aggravating limb ischemia through Wnt5a pathway.Am J Cancer Res. 2024 Apr 15;14(4):1866-1879. doi: 10.62347/SQOC7984. eCollection 2024. Am J Cancer Res. 2024. PMID: 38726275 Free PMC article.
-
Mechanism of engineered macrophage membrane bionic gene-carrying nanospheres for targeted drug delivery to promote wound repair in deep second-degree burns.Sci Rep. 2025 Jan 22;15(1):2756. doi: 10.1038/s41598-025-86716-2. Sci Rep. 2025. PMID: 39843907 Free PMC article.
-
Multimodal roles of transient receptor potential channel activation in inducing pathological tissue scarification.Front Immunol. 2023 Aug 29;14:1237992. doi: 10.3389/fimmu.2023.1237992. eCollection 2023. Front Immunol. 2023. PMID: 37705977 Free PMC article. Review.
References
-
- Monstrey S, Middelkoop E, Vranckx JJ, Bassetto F, Ziegler UE, Meaume S, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67:1017–25. - PubMed
-
- Elsaie ML. Update on management of keloid and hypertrophic scars: a systemic review. J Cosmet Dermatol. 2021;20:2729–38. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

