In-process monitoring of a tissue-engineered oral mucosa fabricated on a micropatterned collagen scaffold: use of optical coherence tomography for quality control
- PMID: 36406717
- PMCID: PMC9667272
- DOI: 10.1016/j.heliyon.2022.e11468
In-process monitoring of a tissue-engineered oral mucosa fabricated on a micropatterned collagen scaffold: use of optical coherence tomography for quality control
Abstract
Background: We previously reported a novel technique for fabricating dermo-epidermal junction (DEJ)-like micropatterned collagen scaffolds to manufacture an ex vivo produced oral mucosa equivalent (EVPOME) for clinical translation; however, more biomimetic micropatterns are required to promote oral keratinocyte-based tissue engineering/regenerative medicine. In addition, in-process monitoring for quality control of tissue-engineered products is key to successful clinical outcomes. However, evaluating three-dimensional tissue-engineered constructs such as EVPOME is challenging. This study aimed to update our technique to fabricate a more biomimetic DEJ structure of oral mucosa and to investigate the efficacy of optical coherence tomography (OCT) in combination with deep learning for non-invasive EVPOME monitoring.
Methods: A picosecond laser-textured microstructure mimicking DEJ on stainless steel was used as a negative mould to fabricate the micropatterned collagen scaffold. During EVPOME manufacturing, OCT was applied twice to monitor the EVPOME and evaluate its epithelial thickness.
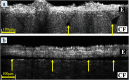
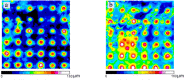
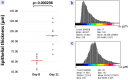
Findings: Our moulding system resulted in successful micropattern replication on the curved collagen scaffold. OCT imaging visualised the epithelial layer and the underlying micropatterned scaffold in EVPOME, enabling to non-invasively detect specific defects not found before the histological examination. Additionally, a gradual increase in epithelial thickness was observed over time.
Conclusion: These findings demonstrate the feasibility of using a stainless-steel negative mould to create a more biomimetic micropattern on collagen scaffolds and the potential of OCT imaging for quality control in oral keratinocyte-based tissue engineering/regenerative medicine.
Keywords: Biomimetics; Micropattern; Optical coherence tomography; Picosecond laser machining; Quality control; Tissue-engineered oral mucosa.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14(3):88–95.
-
- Cheung A. Biomimetic scaffolds for skin and skeletal tissue engineering. J. Biotechnol. Biomater. 2015;5
LinkOut - more resources
Full Text Sources

