Novel ciprofloxacin and norfloxacin-tetrazole hybrids as potential antibacterial and antiviral agents: Targeting S. aureus topoisomerase and SARS-CoV-2-MPro
- PMID: 36406777
- PMCID: PMC9640164
- DOI: 10.1016/j.molstruc.2022.134507
Novel ciprofloxacin and norfloxacin-tetrazole hybrids as potential antibacterial and antiviral agents: Targeting S. aureus topoisomerase and SARS-CoV-2-MPro
Abstract
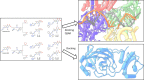
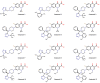
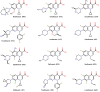
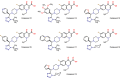
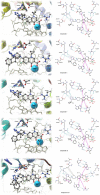
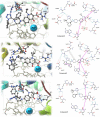
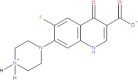
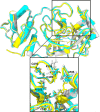
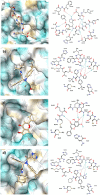
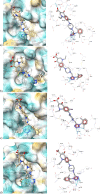
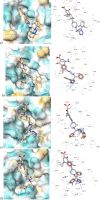
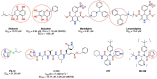
This study was designed to synthesize hybridizing molecules from ciprofloxacin and norfloxacin by enhancing their biological activity with tetrazoles. The synthesized compounds were investigated in the interaction with the target enzyme of fluoroquinolones (DNA gyrase) and COVID-19 main protease using molecular similarity, molecular docking, and QSAR studies. A QSAR study was carried out to explore the antibacterial activity of our compounds over Staphylococcus aureus a QSAR study, using descriptors obtained from the docking with DNA gyrase, in combination with steric type descriptors, was done obtaining suitable statistical parameters ( , , and ) to support our results. The binding interaction of our compounds with CoV-2-Mpro was done by molecular docking and were compared with different covalent and non-covalent inhibitors of this enzyme. For the docking studies we used several crystallographic structures of the CoV-2-Mpro. The interaction energy values and binding mode with several key residues, by our compounds, support the capability of them to be CoV-2-Mpro inhibitors. The characterization of the compounds was completed using FT-IR, 1H-NMR, 13C-NMR, 19F-NMR and HRMS spectroscopic methods. The results showed that compounds 1, 4, 5, 10 and 12 had the potential to be further studied as new antibacterial and antiviral compounds.
Keywords: Covid-19 Main protease; DNA gyrase; Hybrid tetrazole-fluoroquinolone; Molecular docking; Multicomponent reaction; QSAR.
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Molecular Modeling Studies of Novel Fluoroquinolone Molecules.Curr Drug Discov Technol. 2018;15(2):109-122. doi: 10.2174/1570163814666170829142044. Curr Drug Discov Technol. 2018. PMID: 28875852
-
Novel 1,2,4-oxadiazole-chalcone/oxime hybrids as potential antibacterial DNA gyrase inhibitors: Design, synthesis, ADMET prediction and molecular docking study.Bioorg Chem. 2021 Jun;111:104885. doi: 10.1016/j.bioorg.2021.104885. Epub 2021 Apr 1. Bioorg Chem. 2021. PMID: 33838559
-
Design and synthesis of ciprofloxacin-sulfonamide hybrids to manipulate ciprofloxacin pharmacological qualities: Potency and side effects.Eur J Med Chem. 2022 Jan 15;228:114021. doi: 10.1016/j.ejmech.2021.114021. Epub 2021 Nov 30. Eur J Med Chem. 2022. PMID: 34871841
-
Identification of potential plant-based inhibitor against viral proteases of SARS-CoV-2 through molecular docking, MM-PBSA binding energy calculations and molecular dynamics simulation.Mol Divers. 2021 Aug;25(3):1963-1977. doi: 10.1007/s11030-021-10211-9. Epub 2021 Apr 15. Mol Divers. 2021. PMID: 33856591 Free PMC article.
-
Novel Tetrazole-Based Antimicrobial Agents Targeting Clinical Bacteria Strains: Exploring the Inhibition of Staphylococcus aureus DNA Topoisomerase IV and Gyrase.Int J Mol Sci. 2021 Dec 29;23(1):378. doi: 10.3390/ijms23010378. Int J Mol Sci. 2021. PMID: 35008805 Free PMC article.
Cited by
-
Synthesis of tetrazole hybridized with thiazole, thiophene or thiadiazole derivatives, molecular modelling and antimicrobial activity.Saudi Pharm J. 2024 Mar;32(3):101962. doi: 10.1016/j.jsps.2024.101962. Epub 2024 Jan 20. Saudi Pharm J. 2024. PMID: 38318318 Free PMC article.
-
Synthesis, analysis of mol-ecular and crystal structures, estimation of inter-molecular inter-actions and biological properties of 1-benzyl-6-fluoro-3-[5-(4-methylcyclohexyl)-1,2,4-oxadiazol-3-yl]-7-(piperidin-1-yl)quinolin-4-one.Acta Crystallogr E Crystallogr Commun. 2023 Feb 21;79(Pt 3):192-200. doi: 10.1107/S2056989023001305. eCollection 2023 Feb 1. Acta Crystallogr E Crystallogr Commun. 2023. PMID: 36910005 Free PMC article.
-
Novel fluoroquinolones analogues bearing 4-(arylcarbamoyl)benzyl: design, synthesis, and antibacterial evaluation.Mol Divers. 2024 Jun;28(3):1577-1596. doi: 10.1007/s11030-023-10676-w. Epub 2023 Jul 8. Mol Divers. 2024. PMID: 37420079
-
Advancements in Synthetic Strategies and Biological Effects of Ciprofloxacin Derivatives: A Review.Int J Mol Sci. 2024 Apr 30;25(9):4919. doi: 10.3390/ijms25094919. Int J Mol Sci. 2024. PMID: 38732134 Free PMC article. Review.
-
Repositioning of Antibiotics in the Treatment of Viral Infections.Curr Microbiol. 2024 Oct 26;81(12):427. doi: 10.1007/s00284-024-03948-7. Curr Microbiol. 2024. PMID: 39460768 Free PMC article. Review.
References
-
- Castro W., Navarro M., Biot C. Medicinal potential of ciprofloxacin and its derivatives. Future Med. Chem. 2013;5(1):81–96. - PubMed
-
- Luan B., Huynh T., Cheng X., Lan G., Wang H.R. Targeting proteases for treating COVID-19. J. Proteome Res. 2020;19(11):4316–4326. - PubMed
-
- Gil C., et al. COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020;63(21):12359–12386. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

