Cost-Effectiveness Analysis of Transplantation-Ineligible Elderly Patients With Acute Leukemia Harboring a Molecular Target: Ph-Positive Acute Leukemia and FLT3-Mutated Acute Myeloid Leukemia
- PMID: 36406948
- PMCID: PMC9635805
- DOI: 10.14740/jocmr4799
Cost-Effectiveness Analysis of Transplantation-Ineligible Elderly Patients With Acute Leukemia Harboring a Molecular Target: Ph-Positive Acute Leukemia and FLT3-Mutated Acute Myeloid Leukemia
Abstract
Background: Tyrosine kinase inhibitors (TKIs) and FMS-like tyrosine kinase 3 (FLT3) inhibitors are promising agents for Ph-positive acute leukemia (Ph+ AL) and FLT3 mutated acute myeloid leukemia (FLT3-AML), respectively.
Methods: We examined the cost-effectiveness ratio (CER) of dasatinib and ponatinib for Ph+ AL and the cost-effectiveness of gilteritinib and quizartinib for FLT3-AML in elderly patients. Molecular therapy can fit the elderly population better than chemotherapy (CT).
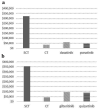
Results: The daily drug cost of dasatinib, ponatinib, gilteritinib, and quizartinib was $240, $170, $524, and $479 in terms of treatment maintenance dose, respectively. Treatment of Ph+ AL with stem cell transplantation (SCT), CT, dasatinib, and ponatinib yielded CERs of $322,375, $34,928, $61,104, and $46,234, respectively. The CERs for FLT3-AML treated with SCT, CT, gilteritinib, and quizartinib were $355,270, $42,717, $94,987, and $90,080, respectively. Treatment of elderly patients with TKIs and FLT3 inhibitors remained expensive and inferior to conventional CT.
Conclusion: Although TKIs and FLT3 inhibitors have an inferior CER than does conventional CT, their promising survival benefit with better QOL can offer a profound advantage. TKI or FLT3 inhibitor monotherapy is recommended as an alternative treatment option for unfit (vulnerable) elderly patients with Ph+ AL or FLT3-AML.
Keywords: Cost-effectiveness analysis; FLT3-mutated acute myeloid leukemia; Health economic evaluation; Ph-positive acute leukemia; Transplantation-ineligible elderly.
Copyright 2022, Imataki et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Emerging FLT3 inhibitors for the treatment of acute myeloid leukemia.Expert Opin Emerg Drugs. 2022 Mar;27(1):1-18. doi: 10.1080/14728214.2021.2009800. Epub 2022 Jan 25. Expert Opin Emerg Drugs. 2022. PMID: 35076348
-
The role of FLT3 inhibitors in the treatment of FLT3-mutated acute myeloid leukemia.Eur J Haematol. 2017 Apr;98(4):330-336. doi: 10.1111/ejh.12841. Epub 2017 Jan 19. Eur J Haematol. 2017. PMID: 28000291 Review.
-
Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia.Biomark Res. 2019 Sep 11;7:19. doi: 10.1186/s40364-019-0170-2. eCollection 2019. Biomark Res. 2019. PMID: 31528345 Free PMC article. Review.
-
Gilteritinib for the treatment of relapsed and/or refractory FLT3-mutated acute myeloid leukemia.Expert Rev Clin Pharmacol. 2019 Sep;12(9):841-849. doi: 10.1080/17512433.2019.1657009. Epub 2019 Aug 27. Expert Rev Clin Pharmacol. 2019. PMID: 31454267 Review.
-
[FLT3 inhibitors in the treatment of FLT3-mutated acute myeloid leukemia].Rinsho Ketsueki. 2021;62(8):954-966. doi: 10.11406/rinketsu.62.954. Rinsho Ketsueki. 2021. PMID: 34497236 Japanese.
Cited by
-
Simvastatin Preferentially Targets FLT3/ITD Acute Myeloid Leukemia by Inhibiting MEK/ERK and p38-MAPK Signaling Pathways.Drugs R D. 2023 Dec;23(4):439-451. doi: 10.1007/s40268-023-00442-6. Epub 2023 Oct 17. Drugs R D. 2023. PMID: 37847357 Free PMC article.
References
-
- Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, Kramer A. et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984–997. doi: 10.1016/S1470-2045(19)30150-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
