Elevated Ca2+ at the triad junction underlies dysregulation of Ca2+ signaling in dysferlin-null skeletal muscle
- PMID: 36406982
- PMCID: PMC9669649
- DOI: 10.3389/fphys.2022.1032447
Elevated Ca2+ at the triad junction underlies dysregulation of Ca2+ signaling in dysferlin-null skeletal muscle
Abstract
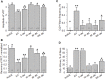
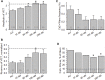
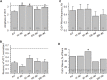
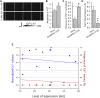
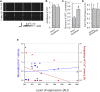
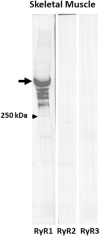
Dysferlin-null A/J myofibers generate abnormal Ca2+ transients that are slightly reduced in amplitude compared to controls. These are further reduced in amplitude by hypoosmotic shock and often appear as Ca2+ waves (Lukyanenko et al., J. Physiol., 2017). Ca2+ waves are typically associated with Ca2+-induced Ca2+ release, or CICR, which can be myopathic. We tested the ability of a permeable Ca2+ chelator, BAPTA-AM, to inhibit CICR in injured dysferlin-null fibers and found that 10-50 nM BAPTA-AM suppressed all Ca2+ waves. The same concentrations of BAPTA-AM increased the amplitude of the Ca2+ transient in A/J fibers to wild type levels and protected transients against the loss of amplitude after hypoosmotic shock, as also seen in wild type fibers. Incubation with 10 nM BAPTA-AM led to intracellular BAPTA concentrations of ∼60 nM, as estimated with its fluorescent analog, Fluo-4AM. This should be sufficient to restore intracellular Ca2+ to levels seen in wild type muscle. Fluo-4AM was ∼10-fold less effective than BAPTA-AM, however, consistent with its lower affinity for Ca2+. EGTA, which has an affinity for Ca2+ similar to BAPTA, but with much slower kinetics of binding, was even less potent when introduced as the -AM derivative. By contrast, a dysferlin variant with GCaMP6fu in place of its C2A domain accumulated at triad junctions, like wild type dysferlin, and suppressed all abnormal Ca2+ signaling. GCaMP6fu introduced as a Venus chimera did not accumulate at junctions and failed to suppress abnormal Ca2+ signaling. Our results suggest that leak of Ca2+ into the triad junctional cleft underlies dysregulation of Ca2+ signaling in dysferlin-null myofibers, and that dysferlin's C2A domain suppresses abnormal Ca2+ signaling and protects muscle against injury by binding Ca2+ in the cleft.
Keywords: BAPTA; CICR; Ca-induced Ca release; GCaMP; dysferlinopathy; injury; osmotic shock.
Copyright © 2022 Lukyanenko, Muriel, Garman, Breydo and Bloch.
Conflict of interest statement
LB is employed by Regeneron Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

