Phosphorylation and regulation of group II metabotropic glutamate receptors (mGlu2/3) in neurons
- PMID: 36407098
- PMCID: PMC9669598
- DOI: 10.3389/fcell.2022.1022544
Phosphorylation and regulation of group II metabotropic glutamate receptors (mGlu2/3) in neurons
Abstract
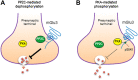
Group II metabotropic glutamate (mGlu) receptors (mGlu2/3) are Gαi/o-coupled receptors and are primarily located on presynaptic axonal terminals in the central nervous system. Like ionotropic glutamate receptors, group II mGlu receptors are subject to regulation by posttranslational phosphorylation. Pharmacological evidence suggests that several serine/threonine protein kinases possess the ability to regulate mGlu2/3 receptors. Detailed mapping of phosphorylation residues has revealed that protein kinase A (PKA) phosphorylates mGlu2/3 receptors at a specific serine site on their intracellular C-terminal tails in heterologous cells or neurons, which underlies physiological modulation of mGlu2/3 signaling. Casein kinases promote mGlu2 phosphorylation at a specific site. Tyrosine protein kinases also target group II receptors to induce robust phosphorylation. A protein phosphatase was found to specifically bind to mGlu3 receptors and dephosphorylate the receptor at a PKA-sensitive site. This review summarizes recent progress in research on group II receptor phosphorylation and the phosphorylation-dependent regulation of group II receptor functions. We further explore the potential linkage of mGlu2/3 phosphorylation to various neurological and neuropsychiatric disorders, and discuss future research aimed at analyzing novel biochemical and physiological properties of mGlu2/3 phosphorylation.
Keywords: PKA; PKC; casein kinase; glutamate; mGlu; phosphatase; phosphorylation.
Copyright © 2022 Mao, Mathur, Mahmood, Rajan, Chu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aronica E., Gorter J. A., Ijlst-Keizers H., Rozemuller A. J., Yankaya B., Leenstra S., et al. (2003). Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: Opposite regulation of glutamate transporter proteins. Eur. J. Neurosci. 17, 2106–2118. 10.1046/j.1460-9568.2003.02657.x - DOI - PubMed
-
- Cai Z., Saugstad J. A., Sorensen S. D., Ciombor K. J., Zhang C., Schaffhauser H., et al. (2001). Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. J. Neurochem. 78, 756–766. 10.1046/j.1471-4159.2001.00468.x - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

