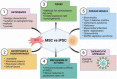
MSCs vs. iPSCs: Potential in therapeutic applications
- PMID: 36407112
- PMCID: PMC9666898
- DOI: 10.3389/fcell.2022.1005926
MSCs vs. iPSCs: Potential in therapeutic applications
Abstract
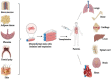
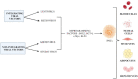
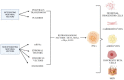
Over the past 2 decades, mesenchymal stem cells (MSCs) have attracted a lot of interest as a unique therapeutic approach for a variety of diseases. MSCs are capable of self-renewal and multilineage differentiation capacity, immunomodulatory, and anti-inflammatory properties allowing it to play a role in regenerative medicine. Furthermore, MSCs are low in tumorigenicity and immune privileged, which permits the use of allogeneic MSCs for therapies that eliminate the need to collect MSCs directly from patients. Induced pluripotent stem cells (iPSCs) can be generated from adult cells through gene reprogramming with ectopic expression of specific pluripotency factors. Advancement in iPS technology avoids the destruction of embryos to make pluripotent cells, making it free of ethical concerns. iPSCs can self-renew and develop into a plethora of specialized cells making it a useful resource for regenerative medicine as they may be created from any human source. MSCs have also been used to treat individuals infected with the SARS-CoV-2 virus. MSCs have undergone more clinical trials than iPSCs due to high tumorigenicity, which can trigger oncogenic transformation. In this review, we discussed the overview of mesenchymal stem cells and induced pluripotent stem cells. We briefly present therapeutic approaches and COVID-19-related diseases using MSCs and iPSCs.
Keywords: COVID-19; SARS-CoV-2; induced pluripotent stem cells; mesenchymal stem cells; therapeutic applications.
Copyright © 2022 Thanaskody, Jusop, Tye, Wan Kamarul Zaman, Dass and Nordin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Al-Anazi K. A. (2020). “Introductory chapter,” in Update on mesenchymal and induced pluripotent stem cells. London: IntechOpen. 10.5772/intechopen.90236 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

