Effect of dietary protein content shift on aging in elderly rats by comprehensive quantitative score and metabolomics analysis
- PMID: 36407526
- PMCID: PMC9673908
- DOI: 10.3389/fnut.2022.1051964
Effect of dietary protein content shift on aging in elderly rats by comprehensive quantitative score and metabolomics analysis
Abstract
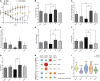
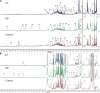
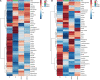
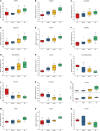
In the protein nutrition strategy of middle-aged and elderly people, some believe that low protein is good for health, while others believe high protein is good for health. Facing the contradictory situation, the following hypothesis is proposed. There is a process of change from lower to higher ratio of protein nutritional requirements that are good for health in the human body after about 50 years of age, and the age at which the switch occurs is around 65 years of age. Hence, in this study, 50, 25-month-old male rats were randomly divided into five groups: Control (basal diet), LP (low-protein diet with a 30% decrease in protein content compared to the basal diet), HP (high-protein diet with a 30% increase in protein content compared to the basal diet), Model 1 (switched from LP to HP feed at week 4), and Model 2 (switched from LP to HP feed at week 7). After a total of 10 weeks intervention, the liver and serum samples were examined for aging-related indicators, and a newly comprehensive quantitative score was generated using principal component analysis (PCA). The effects of the five protein nutritional modalities were quantified in descending order: Model 1 > HP > LP > Control > Model 2. Furthermore, the differential metabolites in serum and feces were determined by orthogonal partial least squares discriminant analysis, and 15 differential metabolites, significantly associated with protein intake, were identified by Spearman's correlation analysis (p < 0.05). Among the fecal metabolites, 10 were positively correlated and 3 were negatively correlated. In the serum, tyrosine and lactate levels were positively correlated, and acetate levels were negatively correlated. MetaboAnalyst analysis identified that the metabolic pathways influenced by protein intake were mainly related to amino acid and carbohydrate metabolism. The results of metabolomic analysis elucidate the mechanisms underlying the preceding effects to some degree. These efforts not only contribute to a unified protein nutrition strategy but also positively impact the building of a wiser approach to protein nutrition, thereby helping middle-aged and older populations achieve healthy aging.
Keywords: aging; comprehensive quantitative score; dietary patterns; metabolomics; nuclear magnetic resonance.
Copyright © 2022 Zheng, Li, Zhou, Shi, Song, Liao, Zhou, Zheng, Lv and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

