Effect of dietary resveratrol on placental function and reproductive performance of late pregnancy sows
- PMID: 36407549
- PMCID: PMC9673905
- DOI: 10.3389/fnut.2022.1001031
Effect of dietary resveratrol on placental function and reproductive performance of late pregnancy sows
Abstract
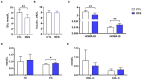
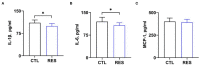
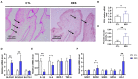
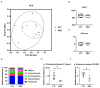
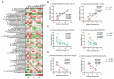
Placental function is vital to the fetal growth of sows, and resveratrol (RES) can protect cells against oxidative stress, which is one of the major factors impairing placental function. This study aimed to investigate the effect of dietary resveratrol (RES) on placental function and reproductive performance during late pregnancy in a sow model from the aspects of oxidative stress, insulin resistance, and gut microbiota. A total of 26 hybrid pregnant sows (Landrace × Yorkshire) with similar parity were randomly allocated into two groups (n = 13) and fed with a basal diet or a diet containing 200 mg/kg of resveratrol from day 85 of gestation until parturition. The dietary supplementation of RES increased the litter weight at parturition by 12.53% (p = 0.145), with ameliorated insulin resistance (HOMA-IR), increased triglyceride (TG) levels, and decreased interleukin (IL)-1β and IL-6 levels in serum (p < 0.05). Moreover, resveratrol increased the placental vascular density (p < 0.05) with the enhanced expression of nutrient transporter genes (SLC2A1 and SLC2A3) and antioxidant genes, such as superoxide dismutase 2 (SOD2) and heme oxygenase-1 (HO-1) but declined the expression of inflammatory genes, such as IL-1β and IL-6 (p < 0.05). The characterization of the fecal microbiota revealed that resveratrol decreased the relative abundance of the Christensensllaceae R-7 group and Ruminococcaceae UCG-008 (p < 0.05), which had a positive linear correlation with the expression of IL-1β and IL-6 (p < 0.05), but had a negative linear correlation with the expression of SOD2, HO-1, SLC2A1, and SCL2A3 genes (p < 0.05). These data demonstrated that dietary supplementation with resveratrol can improve placental function with ameliorated insulin resistance, oxidative stress, and inflammation potentially by regulating Ruminococcaceae UCG-008 and the Christensensllaceae R-7 group in sows.
Keywords: gut microbiota; placental function; reproductive performance; resveratrol; sow.
Copyright © 2022 Hu, Tan, Li, Wang, Shi, Li, Liu, Yuan, He and Wu.
Conflict of interest statement
Author XY was employed by Hunan Xinguang'an Agricultural Husbandry Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

