Is allelochemical synthesis in Casuarina equisetifolia plantation related to litter microorganisms?
- PMID: 36407626
- PMCID: PMC9666782
- DOI: 10.3389/fpls.2022.1022984
Is allelochemical synthesis in Casuarina equisetifolia plantation related to litter microorganisms?
Abstract
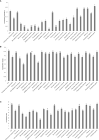
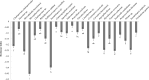
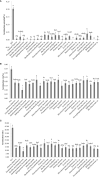
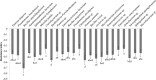
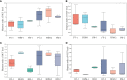
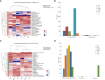
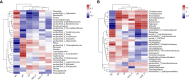
Productivity decline of Casuarina equisetifolia plantation and difficulty in natural regeneration remains a serious problem because of allelopathy. Previous studies have confirmed that 2,4-di-tert-butylphenol (2,4-DTBP) are the major allelochemicals of the C. equisetifolia litter exudates. The production of these allelochemicals may derive from decomposition of litter or from the litter endophyte and microorganisms adhering to litter surfaces. In the present study, we aimed to evaluate the correlation between allelochemicals in litter and endophytic and epiphytic fungi and bacteria from litter. A total of 100 fungi and 116 bacteria were isolated from the interior and surface of litter of different forest ages (young, half-mature, and mature plantation). Results showed that the fermentation broth of fungal genera Mycosphaerella sp. and Pestalotiopsis sp., and bacterial genera Bacillus amyloliquefaciens, Burkholderia-Paraburkholderia, and Pantoea ananatis had the strongest allelopathic effect on C. equisetifolia seeds. Allelochemicals, such as 2,4-DTBP and its analogs were identified in the fermentation broths of these microorganisms using GC/MS analysis. These results indicate that endophytic and epiphytic fungi and bacteria in litters are involved in the synthesis of allelochemicals of C. equisetifolia. To further determine the abundance of the allelopathic fungi and bacteria, Illumina MiSeq high-throughput sequencing was performed. The results showed that bacterial genera with strong allelopathic potential were mainly distributed in the young and half-mature plantation with low abundance, while the abundance of fungal genera Mycosphaerella sp. and Pestalotiopsis sp. were higher in the young and mature plantations. In particular, the abundance of Mycosphaerella sp. in the young and mature plantations were 501.20% and 192.63% higher than in the half-mature plantation, respectively. Overall, our study demonstrates that the litter fungi with higher abundance in the young and mature plantation were involved in the synthesis of the allelochemical 2,4-DTBP of C. equisetifolia. This finding may be important for understanding the relationship between autotoxicity and microorganism and clarifying the natural regeneration problem of C. equisetifolia.
Keywords: 2; 4-DTBP; Casuarina equisetifolia; allelopathy; litter microbial community; microbial metabolites.
Copyright © 2022 Xu, Zuo, Zhang, Huang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Endophytic bacteria with allelopathic potential regulate gene expression and metabolite production in host Casuarina equisetifolia.Front Plant Sci. 2024 Sep 18;15:1435440. doi: 10.3389/fpls.2024.1435440. eCollection 2024. Front Plant Sci. 2024. PMID: 39359630 Free PMC article.
-
[Diversity of bacteria and allelopathic potential of their metabolites in differently aged Casuarina equisetifolia litter].Ying Yong Sheng Tai Xue Bao. 2020 Jul;31(7):2185-2194. doi: 10.13287/j.1001-9332.202007.037. Ying Yong Sheng Tai Xue Bao. 2020. PMID: 32715680 Chinese.
-
Root exudates and chemotactic strains mediate bacterial community assembly in the rhizosphere soil of Casuarina equisetifolia L.Front Plant Sci. 2022 Sep 20;13:988442. doi: 10.3389/fpls.2022.988442. eCollection 2022. Front Plant Sci. 2022. PMID: 36212345 Free PMC article.
-
Allelopathic bacteria and their impact on higher plants.Crit Rev Microbiol. 2001;27(1):41-55. doi: 10.1080/20014091096693. Crit Rev Microbiol. 2001. PMID: 11305367 Review.
-
Allelopathic Plants: Models for Studying Plant-Interkingdom Interactions.Trends Plant Sci. 2020 Feb;25(2):176-185. doi: 10.1016/j.tplants.2019.11.004. Epub 2019 Dec 11. Trends Plant Sci. 2020. PMID: 31837955 Review.
Cited by
-
Editorial: Mechanisms and practices for the management of plant-soil biota interaction.Front Plant Sci. 2024 Apr 9;15:1399420. doi: 10.3389/fpls.2024.1399420. eCollection 2024. Front Plant Sci. 2024. PMID: 38654906 Free PMC article. No abstract available.
-
Endophytic bacteria with allelopathic potential regulate gene expression and metabolite production in host Casuarina equisetifolia.Front Plant Sci. 2024 Sep 18;15:1435440. doi: 10.3389/fpls.2024.1435440. eCollection 2024. Front Plant Sci. 2024. PMID: 39359630 Free PMC article.
References
-
- Bi B. Y., Yuan Y., Zhang H., Wu Z. H., Wang Y., Han F. P. (2022). Rhizosphere soil metabolites mediated microbial community changes of Pinus sylvestris var. mongolica across stand ages in the mu us desert. Appl. Soil Ecol. 169, 104222. doi: 10.1016/j.apsoil.2021.104222 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

