Multi-omics analysis reveals neuroinflammation, activated glial signaling, and dysregulated synaptic signaling and metabolism in the hippocampus of aged mice
- PMID: 36408109
- PMCID: PMC9669972
- DOI: 10.3389/fnagi.2022.964429
Multi-omics analysis reveals neuroinflammation, activated glial signaling, and dysregulated synaptic signaling and metabolism in the hippocampus of aged mice
Abstract
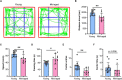
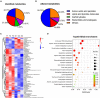
Aging is an intricate biological event that occurs in both vertebrates and invertebrates. During the aging process, the brain, a vulnerable organ, undergoes structural and functional alterations, resulting in behavioral changes. The hippocampus has long been known to be critically associated with cognitive impairment, dementia, and Alzheimer's disease during aging; however, the underlying mechanisms remain largely unknown. In this study, we hypothesized that altered metabolic and gene expression profiles promote the aging process in the hippocampus. Behavioral tests showed that exploration, locomotion, learning, and memory activities were reduced in aged mice. Metabolomics analysis identified 69 differentially abundant metabolites and showed that the abundance of amino acids, lipids, and microbiota-derived metabolites (MDMs) was significantly altered in hippocampal tissue of aged animals. Furthermore, transcriptomic analysis identified 376 differentially expressed genes in the aged hippocampus. A total of 35 differentially abundant metabolites and 119 differentially expressed genes, constituting the top 200 correlations, were employed for the co-expression network. The multi-omics analysis showed that pathways related to inflammation, microglial activation, synapse, cell death, cellular/tissue homeostasis, and metabolism were dysregulated in the aging hippocampus. Our data revealed that metabolic perturbations and gene expression alterations in the aged hippocampus were possibly linked to their behavioral changes in aged mice; we also provide evidence that altered MDMs might mediate the interaction between gut and brain during the aging process.
Keywords: brain ageing; learning and memory; metabolomics; microbiota-derived metabolite; multi-omics analysis; neuroinflammation; synaptic plasticity; transcriptomics.
Copyright © 2022 Lu, Xu, Lin, Wang, Fu, Deng, Croppi and Zhang.
Conflict of interest statement
Author GC was employed by Connect Biopharma Ltd. (Taicang). The remaining authors declare that the research was conducted without any commercial or financial relationships construed as a potential conflict of interest.
Figures




References
-
- Agus A., Planchais J., Sokol H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23 716–724. - PubMed
LinkOut - more resources
Full Text Sources

