Redox signaling and metabolism in Alzheimer's disease
- PMID: 36408110
- PMCID: PMC9670316
- DOI: 10.3389/fnagi.2022.1003721
Redox signaling and metabolism in Alzheimer's disease
Abstract
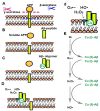
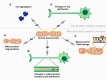
Reduction and oxidation reactions are essential for biochemical processes. They are part of metabolic pathways and signal transduction. Reactive oxygen species (ROS) as second messengers and oxidative modifications of cysteinyl (Cys) residues are key to transduce and translate intracellular and intercellular signals. Dysregulation of cellular redox signaling is known as oxidative distress, which has been linked to various pathologies, including neurodegeneration. Alzheimer's disease (AD) is a neurodegenerative pathology linked to both, abnormal amyloid precursor protein (APP) processing, generating Aβ peptide, and Tau hyperphosphorylation and aggregation. Signs of oxidative distress in AD include: increase of ROS (H2O2, O2 •-), decrease of the levels or activities of antioxidant enzymes, abnormal oxidation of macromolecules related to elevated Aβ production, and changes in mitochondrial homeostasis linked to Tau phosphorylation. Interestingly, Cys residues present in APP form disulfide bonds that are important for intermolecular interactions and might be involved in the aggregation of Aβ. Moreover, two Cys residues in some Tau isoforms have been shown to be essential for Tau stabilization and its interaction with microtubules. Future research will show the complexities of Tau, its interactome, and the role that Cys residues play in the progression of AD. The specific modification of cysteinyl residues in redox signaling is also tightly connected to the regulation of various metabolic pathways. Many of these pathways have been found to be altered in AD, even at very early stages. In order to analyze the complex changes and underlying mechanisms, several AD models have been developed, including animal models, 2D and 3D cell culture, and ex-vivo studies of patient samples. The use of these models along with innovative, new redox analysis techniques are key to further understand the importance of the redox component in Alzheimer's disease and the identification of new therapeutic targets in the future.
Keywords: APP; Alzheimer's disease; Tau; neurodegeneration; redox metabolism; redox signaling.
Copyright © 2022 Holubiec, Gellert and Hanschmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

