Anticancer effects of ABTL0812, a clinical stage drug inducer of autophagy-mediated cancer cell death, in glioblastoma models
- PMID: 36408162
- PMCID: PMC9668006
- DOI: 10.3389/fonc.2022.943064
Anticancer effects of ABTL0812, a clinical stage drug inducer of autophagy-mediated cancer cell death, in glioblastoma models
Erratum in
-
Corrigendum: Anticancer effects of ABTL0812, a clinical stage drug inducer of autophagy-mediated cancer cell death, in glioblastoma models.Front Oncol. 2025 Jan 22;15:1538834. doi: 10.3389/fonc.2025.1538834. eCollection 2025. Front Oncol. 2025. PMID: 39911630 Free PMC article.
Abstract
Background: Glioblastoma multiforme (GBM) is the most malignant adult brain tumor. Current standard of care treatments have very limited efficacy, being the patients´ overall survival 14 months and the 2-year survival rate less than 10%. Therefore, the treatment of GBM is an urgent unmet clinical need.
Methods: The aim of this study was to investigate in vitro and in vivo the potential of ABTL0812, an oral anticancer compound currently in phase II clinical stage, as a novel therapy for GBM.
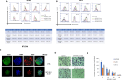
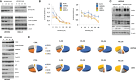
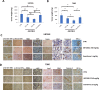
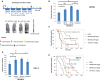
Results: We showed that ABTL0812 inhibits cell proliferation in a wide panel of GBM cell lines and patient-derived glioblastoma stem cells (GSCs) with half maximal inhibitory concentrations (IC50s) ranging from 15.2 µM to 46.9 µM. Additionally, ABTL0812 decreased GSCs neurosphere formation. GBM cells aggressiveness is associated with a trans-differentiation process towards a less differentiated phenotype known as proneural to mesenchymal transition (PMT). ABTL0812 was shown to revert PMT and induce cell differentiation to a less malignant phenotype in GBM cell lines and GSCs, and consequently reduced cell invasion. As previously shown in other cancer types, we demonstrated that the molecular mechanism of action of ABTL0812 in glioblastoma involves the inhibition of Akt/mTORC1 axis by overexpression of TRIB3, and the activation of endoplasmic reticulum (ER) stress/unfolded protein response (UPR). Both actions converge to induce autophagy-mediated cell death. ABTL0812 anticancer efficacy was studied in vivo using subcutaneous and orthotopic intra-brain xenograft tumor models. We demonstrated that ABTL0812 impairs tumor growth and increases disease-free survival and overall survival of mice. Furthermore, the histological analysis of tumors indicated that ABTL0812 decreases angiogenesis. Finally, we investigated the combination of ABTL0812 with the standard of care treatments for GBM radiotherapy and temozolomide in an orthotopic model, detecting that ABTL0812 potentiates the efficacy of both treatments and that the strongest effect is obtained with the triple combination of ABTL0812+radiotherapy+temozolomide.
Conclusions: Overall, the present study demonstrated the anticancer efficacy of ABTL0812 as single agent and in combination with the GBM standard of care treatments in models of glioblastoma and supports the clinical investigation of ABTL0812 as a potential novel therapy for this aggressive brain tumor type.
Keywords: ABTL0812; Akt; ER stress; TRIB3; UPR; autophagy; glioblastoma; mTOR.
Copyright © 2022 Mancini, Colapietro, Cristiano, Rossetti, Mattei, Gravina, Perez-Montoyo, Yeste-Velasco, Alfon, Domenech and Festuccia.
Conflict of interest statement
HP-M, MY-V, JA, and CD are employees of Ability Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

